Environmental Engineering Reference
In-Depth Information
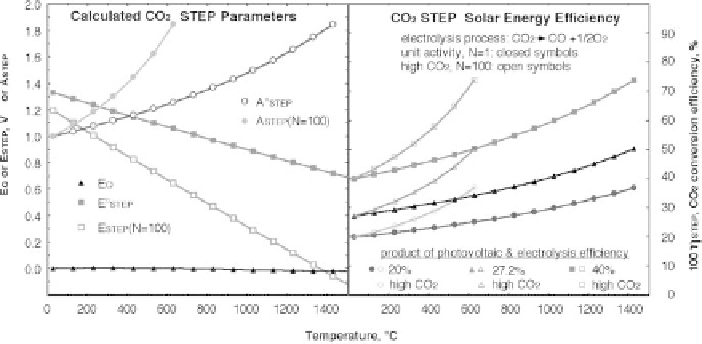
The example of E
STEP
(T,a
100,
and results in a substantial drop in the energy to split CO
2
due to the discussed influ-
ence of RT/2F. Note that at high temperature conditions in the figure, E
STEP
<
0 occurs,
denoting the state in which the reactants are spontaneously formed (without an applied
potential). This could lead to the direct thermochemical generation of products, but
imposes substantial experimental challenges. To date, analogous direct water-splitting
attempts are highly inefficient due to the twin challenges of high temperature mate-
rial constraints and the difficulty in product separation to prevent back reaction upon
cooling (Kogan, 1998). The
STEP
process avoids this back reaction through the sep-
aration of products, which spontaneously occurs in the electrochemical, rather than
chemical, generation of products at separate anode and cathode electrodes.
The differential heat required for CO
2
splitting, E
Q
, and the potential at unit
activity, E
◦
STEP
, are calculated and presented in the top of Figure 8.4.1. E
Q
has also
been calculated and is included. E
Q
is small (comprising tens of millivolts or less)
over the entire temperature range. Hence from Equation 8.4.2, E
STEP
does not differ
significantly from the values presented for E
T
for CO
2
in Figure 8.2.2. E
CO2split
(25
◦
C)
yields A
STEP
(T)
=
1) on the left side of Figure 8.4.1 is derived when N
=
1.333 V/E
STEP
(T) with unit activity, and A
STEP
(T)
=
=
1.197 V/E
STEP
(T)
for the N
100 case. Large resultant
STEP
factors are evident in the left of Figure 8.4.1.
This generates substantial values of solar to chemical energy conversion efficiency for
the
STEP
CO
2
splitting to CO and O
2
.
A
STEP
process operating in the
=
η
PV
· η
electrolysis
range of 0.20 to 0.40 includes the
range of contemporary 25 to 45% efficient concentrator photovoltaics, (King et al.,
2007; Green et al., 2011) and electrolysis efficiency range of 80 to 90%. From these,
the CO
2
solar splitting efficiencies are derived from Equations 8.4.4 and 8.4.5, and
are summarized on the right side of Figure 8.4.1. The small values of E
STEP
(T) at
Figure 8.4.1
Top: Calculated
STEP
parameters for the solar conversion of CO
2
. Bottom: Solar to
chemical conversion efficiencies calculated through Equation 8.4.4 for the conversion of
CO
2
to CO and O
2
. In the case in which the product of the photovoltaic and electrolysis
efficiency is 27.2% (
η
PV
· η
electrolysis
=
0.272), the
STEP
conversion efficiency at unit activity
is 35%, at the 650
◦
C temperature consistent with molten carbonate electrolysis, rising
to 40% at the temperature consistent with solid oxide electrolysis (1000
◦
C). Non-unit
activity calculations presented are for the case of
√
2a
CO
2
a
−
3
/
2
CO
=
100. A solar conversion
efficiency of 50% is seen at 650
◦
C when N
=
100 (the case of a cell with 1 bar of CO
2
and
∼
58 mbar CO). Modified with permission from Licht 2009.
Search WWH ::

Custom Search