Environmental Engineering Reference
In-Depth Information
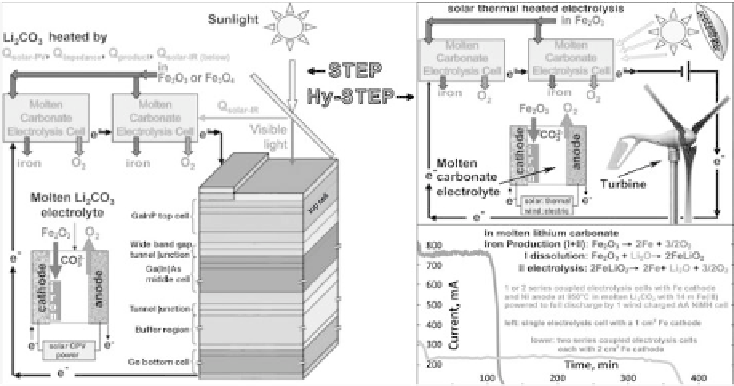
Figure 8.3.7
STEP and (wind) Hy-STEP iron. Left: STEP iron production in which two molten carbonate
electrolysis in series are driven by a concentrator photovoltaic.The 2.7V maximum power
of the CPV can drive either two 1.35V iron electrolyses at 800
◦
C (schematically repre-
sented), or three 0.9V iron electrolyses at 950
◦
C.At 0.9V, rather than at E
◦
(25
◦
C)
=
1.28V,
there is a considerably energy savings, achieved through the application of external heat,
including solar thermal, to the system. Right: The Hy-STEP solar thermal/wind produc-
tion of CO
2
free iron. Concentrated sunlight heats, and wind energy drives electronic
transfer into the electrolysis chamber. The required wind powered electrolysis energy is
diminished by the high temperature and the high solubility of iron oxide. Bottom: Iron is
produced at high current density and low energy at an iron cathode and with a Ni anode
in 14m Fe
2
O
3
+
14m Li
2
O dissolved in molten Li
2
CO
3
. Modified with permission from
Licht et al. 2011b.
a convenient power source to drive the low electrolysis energy iron deposition without
CO
2
formation in Li
2
CO
3
, (Licht, 2009) as schematically represented in Figure 8.3.7.
A solar/wind
Hy
brid
S
olar
T
hermal
E
lectrochemical
P
roduction iron electrolysis
process is also demonstrated (Licht et al., 2011b). In lieu of solar electric, electronic
energy can be provided by alternative renewables, such as wind. As shown on the
right side of Figure 8.3.7, in this Hy-STEP example, the electronic energy is driven by
a wind turbine and concentrated sunlight is only used to provide heat to decrease the
energy required for iron splitting. In this process, sunlight is concentrated to provide
effective heating, but is not split into separate spectral regions as in our alternative
implmentation. Hy-STEP iron production is measured with a 31.5
×
44
.
5
Fresnel lens
(Edmund Optics) which concentrates sunlight to provide temperatures of over 950
◦
C,
and a Sunforce-44444 400 W wind turbine provides electronic charge, charging series
nickel metal hydride, MH, cells at 1.5 V). Each MH cell, provides a constant discharge
potential of 1.0-1.3 V, which are each used to drive one or two series connected iron
electrolysis cells as indicated in the right side of Figure 8.3.7, containing 14 m Fe(III)
molten Li
2
CO
3
electrolysis cells. Electrolysis current is included in the lower right of
Figure 8.3.7. Iron metal is produced. Steel (iron containing carbon) may be directly
formed via the concurrent reduction of CO
2
, as will be delineated in an expanded study.




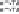
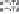























Search WWH ::

Custom Search