Environmental Engineering Reference
In-Depth Information
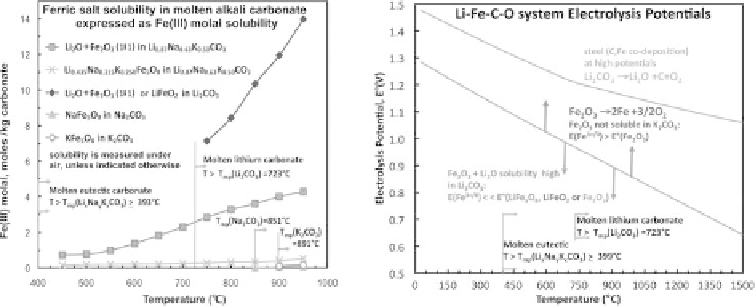
Figure 8.3.6
Left: Measured ferric oxides solubilities in alkali molten carbonates. Right: Calculated
unit activity electrolysis potentials of LiFe
5
O
8
,Fe
2
O
3
or Li
2
CO
3
. Vertical arrows indicate
Nernstian shifts at high or low Fe(III). Modified with permission from Licht and Wang
2010.
Fe(III) solubility is similar when either LiFeO
2
, or LiFeO
2
as Fe
2
O
3
+
Li
2
O, is added
to the Li
2
CO
3
. As seen in the left side of Figure 8.3.6, the solubility of LiFeO
2
is over
12 m above 900C
◦
in Li
2
CO
3
.
Solid reaction of Fe
2
O
3
and Na
2
CO
3
produces both NaFeO
2
and NaFe
5
O
8
prod-
ucts (Lykasov and Pavlovskaya, 2003). As seen in Figure 8.3.6, our unlike Li
2
CO
3
,
measurements in either molten Na
2
CO
3
or K
2
CO
3
, exhibit
<<
1 wt% iron oxide sol-
ubility, even at 950
◦
C. However the solubility of (Li
2
O
Fe
2
O
3
) is high in the alkali
carbonate eutectic, Li
0
.
87
Na
0
.
63
K
0
.
50
CO
3
, and is approximately proportional to the
Li fraction in the pure Li
2
CO
3
electrolyte. Solubility of this lithiated ferric oxide in
the Li
x
Na
y
K
z
CO
3
mixes provides an alternative molten media for iron production,
which compared to pure lithium carbonate, has the disadvantage of lower conductiv-
ity, (Licht and Wang, 2010) but the advantage of even greater availability, and a wider
operating temperature domain range (extending several hundred degrees lower than
the pure lithium system).
Fe
2
O
3
or LiFe
5
O
8
dissolves rapidly in molten Li
2
CO
3
, but reacts with the molten
carbonate as evident in a mass loss, which evolves one equivalent of CO
2
per Fe
2
O
3
,
to form a steady state concentration of LiFeO
2
in accord with the reaction of Equa-
tion 8.3.7 (but occurring in molten carbonate) (Licht et al., 2011b). However, 1
equivalent of Li
2
O and 1 equivalent of Fe
2
O
3
, or LiFeO
2
, dissolves without the reactive
formation of CO
2
. This is significant for the electrolysis of Fe
2
O
3
in molten carbon-
ate. As LiFeO
2
is reduced Li
2
O is released, Equation 8.3.8, facilitating the continued
dissolution of Fe
2
O
3
without CO
2
release or change in the electrolyte, More concisely,
iron production via hematite in Li
2
CO
3
is given by I and II:
+
I
dissolution in molten carbonate: Fe
2
O
3
+
Li
2
O
→
2LiFeO
2
(8.3.7)
II
electrolysis, Li
2
O regeneration: 2LiFeO
2
→
2Fe
+
Li
2
O
+
3
/
2O
2
(8.3.8)
Iron Production
,Li
2
O unchanged(I
+
II): Fe
2
O
3
→
2Fe
+
3
/
2O
2
(8.3.9)
Search WWH ::

Custom Search