Environmental Engineering Reference
In-Depth Information
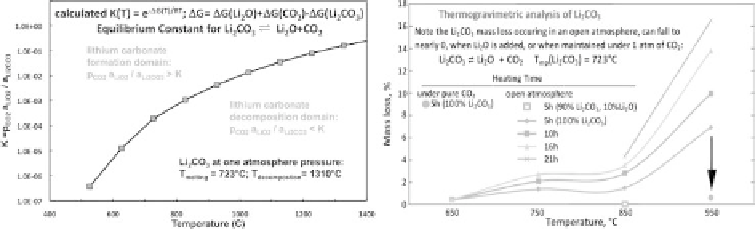
Figure 8.3.4
Left: Species stability in the lithium carbonate, lithium oxide, carbon dioxide system, as
calculated from Li
2
CO
3
,Li
2
O, and CO
2
thermochemical data. Right:Thermogravimetric
analysis of lithium carbonate. The measured mass loss in time of Li
2
CO
3
. Not shown:
The Li
2
CO
3
mass loss rate also decreases with an increasing ratio of Li
2
CO
3
mass to
the surface area of the molten salt exposed to the atmosphere. This increased ratio, may
increase the released partial pressure of CO
2
above the surface, increase the rate of the
back reaction (Li
2
O+CO
2
→
Li
2
CO
3
), and therefore result in the observed decreased
mass loss. Hence, under an open atmosphere at 950
◦
C, the mass loss after 5 hours falls
from 7% to 4.7%, when the starting mass of pure Li
2
CO
3
in the crucible is increased from
20 to 50 g. Under these latter conditions (open atmosphere, 950
◦
C, 50 g total electrolyte),
but using a 95% Li
2
CO
3
,5%Li
2
O mix, the rate of mass loss is only 2.3%. Modified with
permission from Licht et al. 2011a.
As delineated in Section 8.2.3, in practice, either STEP or Hy-STEP modes are
useful for efficient solar carbon capture. CO
2
added to the cell is split at 50% solar
to chemical energy conversion efficiency by series coupled lithium carbonate electrol-
ysis cells driven at maximum power point by an efficient CPC. Experimentally, we
observe the facile reaction of CO
2
and Li
2
O in molten Li
2
CO
3
. We can also calculate
the thermodynamic equilibrium conditions between the species in the system, Equa-
tion 8.2.3B. Using the known thermochemistry of Li
2
O, CO
2
and Li
2
CO
3
, (Chase,
1998) we calculate the reaction free-energy of Equation 8.2.1, and from this calcu-
late the thermodynamic equilibrium constant as a function of temperature. From this
equilibrium constant, the area above the curve on the left side of Figure 8.3.4 presents
the wide domain (above the curve) in which Li
2
CO
3
dominates, that is where excess
CO
2
reacts with Li
2
O such that p
CO2
·
a
Li2O
<
a
Li2CO3
. This is experimentally verified
when we dissolve Li
2
O in molten Li
2
CO
3
, and inject CO
2
(gas). Through the measured
mass gain, we observe the rapid reaction to Li
2
CO
3
. Hence, CO
2
is flowed into a solu-
tion of 5% by weight Li
2
O in molten Li
2
CO
3
at 750
◦
C, the rate of mass gain is only
limited by the flow rate of CO
2
into the cell (using an Omega FMA 5508 mass flow
controller) to react one equivalent of CO
2
per dissolved Li
2
O. As seen in the measured
thermogravimetric analysis on the right side of Figure 8.3.4, the mass loss in time is
low in lithium carbonate heated in an open atmosphere (
0.03% CO
2
)upto850
◦
C,
but accelerates when heated to 950
◦
C. However the 950
◦
C mass loss falls to nearly
zero, when heated under pure (1 atm) CO
2
. Also in accord with Equation 8.2.1 added
Li
2
O shifts the equilibrium to the left. As seen in the figure in an open atmosphere,
there is no mass loss in a 10% Li
2
O, 90% Li
2
CO
3
at 850
◦
C, and the Li
2
O contain-
ing electrolyte absorbs CO
2
(gains mass) at 750
◦
C to provide for the direct carbon
∼
Search WWH ::

Custom Search