Environmental Engineering Reference
In-Depth Information
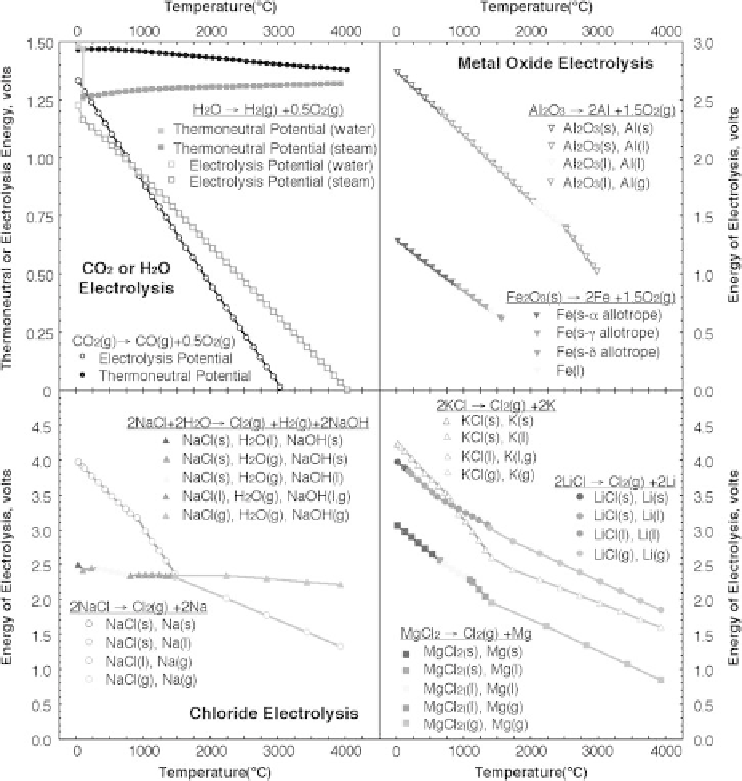
Figure 8.2.1
The calculated potential to electrolyze selected oxides (top) and chlorides (bottom). The
indicated decrease in electrolysis energy, with increase in temperature, provides energy
savings in the
STEP
process in which high temperature is provided by excess solar heat.
Energies of electrolysis are calculated fromEquation 8.2.3,with consistent thermochemical
values at unit activity using NIST gas and condensed phase Shomate equations (Chase,
1998). Note with water excluded, the chloride electrolysis decreases (in the lower left of
the figure). All other indicated electrolysis potentials, including that of water or carbon
dioxide, decrease with increasing temperature. Thermoneutral potentials are calculated
with Equation 8.2.5. Modified with permission from Licht 2009.
η
thermal
is higher than
η
solar-electric
, and gains in efficiency occur in Equation 8.2.6 in the
limit as E
electrolysis
approaches 0. E
electrolysis
=
0 is equivalent to thermochemical, rather
than electrolytic, production. As seen in Figure 8.2.1, at unit activity E
◦
CO2
/
CO
does not
approach 0 until 3000
◦
C. Material constraints inhibit approach to this higher tempera-
ture, while electrolysis also provides the advantage of spontaneous product seperation.
Search WWH ::

Custom Search