Biology Reference
In-Depth Information
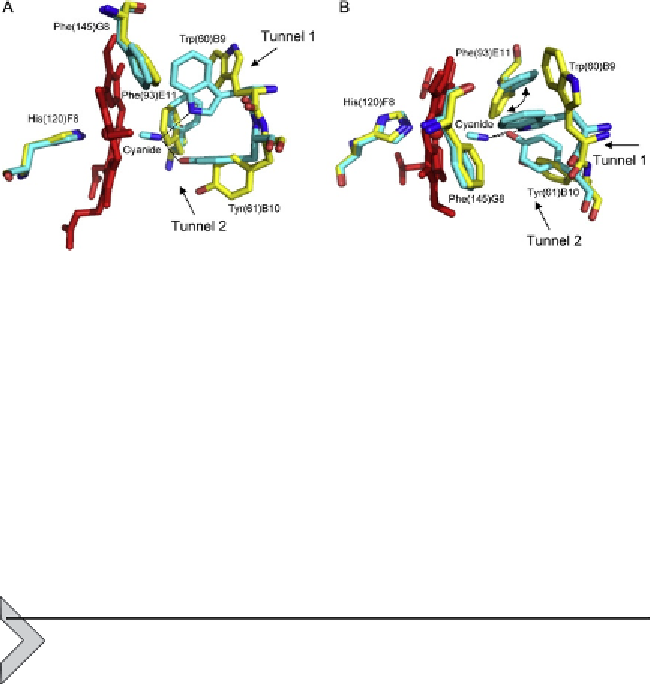
Figure 3.2 The haem distal site of MaPgb
*
. Superimposition of the ferric ligand-free
MaPgb
*
(white) onto the MaPgb
*
(III)-cyanide structure (grey). The haem is shown in
black. Residues lining the haem distal pocket are indicated and shown in stick represen-
tation, together with the proximal His(120)F8 residue. Hydrogen bonds to the haem-Fe
(III)-bound cyanide are indicated by dashed lines. The haem distal cavity entrance sites
of tunnel 1 and tunnel 2 are indicated by arrows. (A) Side view and (B) top view: rotation
of the Phe(93)E11 side chain upon ligand binding is also indicated.
(and mutants) reported so far, where irrespective of the ligation state of the
protein and of the open/closed conformation of tunnel 1 (due to the location
of Trp(60)B9 side chain) Phe(145)G8 is always in the open conformation
(
Fig. 3.2
).
4. THE HAEM ENVIRONMENT
The Pgb haem cavity is characterised by strong hydrophobicity, both
at the proximal and distal sites. Besides the proximal His(120)F8-Fe
coordination bond,
Ma
Pgb
*
-haem interactions include van der Waals con-
tacts with hydrophobic residues at the conserved topological positions:
Ile(116)F4, Thr(128), Ile(137), Leu(142)G4, Trp(185)H17 at the haem-
proximal side, and Trp(60)B9, Tyr(61)B10, Val(64)B13, Phe(74)CD1,
Val(89)E7, Phe(93)E11, Phe(145)G7 and Ile(149)G11 at the haem distal
side. In particular, Phe(74)CD1, Phe(93)E11 and Phe(145)G7 provide
p
-
p
interactions with three out of four porphyrins pyrrole rings. Further
stabilising interactions are provided by hydrogen bonds between the haem
propionates and polar residues, involving Tyr(85)E3, Arg(92)E10, Tyr(112)
and Arg(119)F7, and interaction with a buried water molecule.
The high-resolution crystal structure of
Ma
Pgb
*
provides unequivocal
evidence of substantial distortion in the porphyrin ring system,
likely