Biology Reference
In-Depth Information
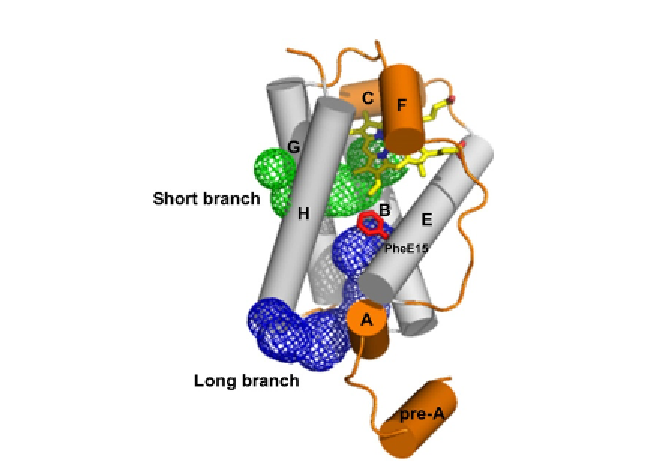
Figure 2.2 The protein matrix tunnel (short and long branches) observed in group I
M. tuberculosis 2/2HbN. The tunnel surface, defined by a 1.4 Å radius probe, is portrayed
as a mesh. Residue PheE15, causing the main restriction in the diameter of the long
branch tunnel, is shown in the close conformation in stick representation and labelled.
Helices are shown as cylinders and labelled. The helices structurally conserved within
3/3 and 2/2 folds are shown in grey. The haem is shown in stick representation.
survival of its host through the rapid oxidation of NO to harmless nitrate
(
Couture, Yeh, et al., 1999; Ouellet et al., 2002; Pathania, Navani,
Gardner, Gardner, & Dikshit, 2002
). Migration of O
2
and NO to the
M. tuberculosis
2/2HbN distal haem cavity is driven by a dual-path mecha-
nism. In fact, by long molecular dynamics simulations (0.1 ms), it has been
shown that in deoxy 2/2HbN, PheE15 adopts the closed conformation and
hence the O
2
ligand enters the protein
via
the short channel. In the case of
oxygenated 2/2HbN, the PheE15 prefers the open conformation, thus facil-
itating entrance of the second ligand (NO)
via
the long tunnel branch
(
Bidon-Chanal et al., 2006; Bidon-Chanal, Mart`, Estrin, & Luque,
2007
). Recent mutagenesis studies also support this view on the diffusion
of small diatomic ligands through the
M. tuberculosis
2/2HbN protein matrix
tunnel system, pointing out the delicate structural balance imposed by the
PheE15 gate, which not only regulates ligand migration but also contributes
to avoid the collapse of helices B and E, thus preserving the ligand accessi-
bility along the tunnel long branch (
Oliveira et al., 2012
).