Biology Reference
In-Depth Information
2. FOLD AND FOLD VARIATION IN 2/2Hb GROUPS I, II, III
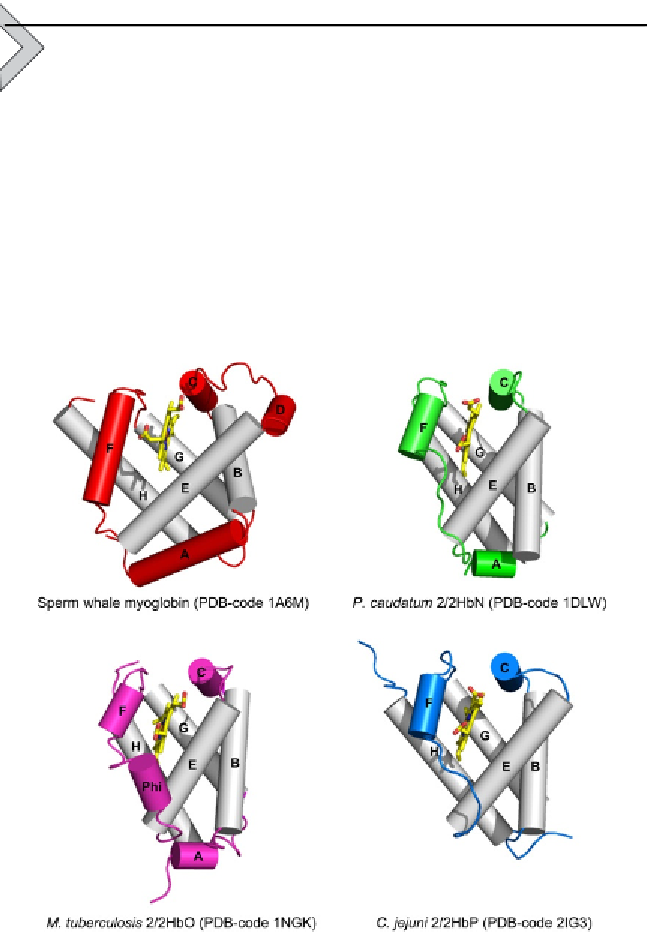
The globin fold of 2/2Hb (
Fig. 2.1
) has been described as consisting of
a simplified version of the 'classical' globin fold (a 3-on-3
a
-helical sand-
wich;
Perutz, 1979
) typical of sperm whale Mb. The topology of the
2/2Hb fold is characterized by a 2-on-2
a
-helical sandwich based on four
a
-helices, corresponding to the B-, E-, G-, and H-helices of the classical
globin fold (
Nardini et al., 2007; Pesce et al., 2000
). The helix pairs B/E
and G/H are arranged each in antiparallel fashion and assembled in a sort
of
a
-helical bundle which surrounds and protects the haem group from
the solvent. Although the G- and H-helices generally match the globin fold
Figure 2.1 Comparative view of the classical 3/3 globin fold (sperm whale myoglobin)
with the 2/2 globin fold in groups I (HbN), II (HbO), and III (HbP). Helices are shown as
cylinders and labelled. The helices structurally conserved within 3/3 and 2/2 folds are
shown in grey. The haem is shown in stick representation.