Biology Reference
In-Depth Information
Hexacoordination of the haem-Fe atom may suggest a common physi-
ological mechanism for protecting cells against oxidative chemistry in
response to high O
2
concentration. Several roles have been hypothesised
for hexacoordinated Ngb and Cygb, for example, as O
2
scavengers under
hypoxic conditions (
Burmester, Ebner, Weich, & Hankeln, 2002;
Burmester, Weich, Reinhardt, & Hankeln, 2000
), as terminal oxidases
(
Sowa, Guy, Sowa, & Hill, 1999
), as O
2
-sensor proteins (
Kriegl et al.,
2002
), and in NO metabolism (
Smagghe et al., 2008
). It was recently
reported that Ngb over-expression and intracellular localisation confer pro-
tection to neurons, both
in vitro
and
in vivo
, against oxidative stress and
enhance cell survival under anoxia and ischaemic conditions (
Fiocchetti,
De Marinis, Ascenzi, & Marino, 2013
). However, their physiological role
is still a matter of debate.
Hexacoordinated globins are characterised by specific electronic
absorption bands in the UV-visible spectra, clearly indicating the electronic
structure of the Fe atom and its axial ligands (
Dewilde et al., 2001
).
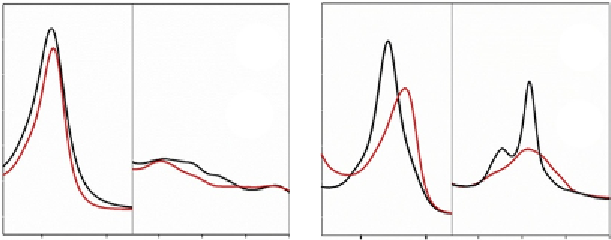
The electronic-absorption spectrum of ferric
Ph
-2/2HbO is
characterised by hexacoordinated high-spin (bands at 503 nm and charge-
transfer transition at 635 nm) and low-spin forms (bands at 533 and
570 nm) (
Fig. 8.6
A), the latter being characteristic of a Tyr coordinated
A
B
X5
X5
400
450
500
550
600
650
400
450
500
550
600
650
Wavelength (nm)
Wavelength (nm)
Figure 8.6 Overlay of absorption spectra of (A) ferric hexacoordinated Ph-2/2HbO
(black line) with ferric Mb (red line) and (B) deoxy ferrous hexacoordinated Ph-2/
2HbO (black line) with ferrous Mb (red line). All measurements were at pH 7.6 and
25
C. The ferrous samples were prepared by adding 2 ml of sodium dithionite
(10 mg ml
1
) to 600 ml of deoxygenated buffered solution of ferric globins, obtained
flushing the ferric forms with nitrogen. The protein concentration was 10 mM on a haem
basis.