Biology Reference
In-Depth Information
Lecomte, 2006; Wittenberg et al., 2002
). The crystal structures of TrHbsII
from
B. subtilis
(
Giangiacomo, Ilari, Boffi, Morea, & Chiancone, 2005
),
T. fusca
(
Bonamore et al., 2005
),
Geobacillus stearothermophilus
(
Ilari et al.,
2007
) and
M. tuberculosis
(
Milani et al., 2005, 2003
) show a network of inter-
actions between polar residues and the haem-Fe atom that may explain the
high O
2
affinity of these globins (
Bonamore et al., 2005; Giangiacomo et al.,
2005; Ilari et al., 2007; Milani et al., 2005; Mukai, Savard, Ouellet,
Guertin, & Yeh, 2002; Ouellet et al., 2003
).
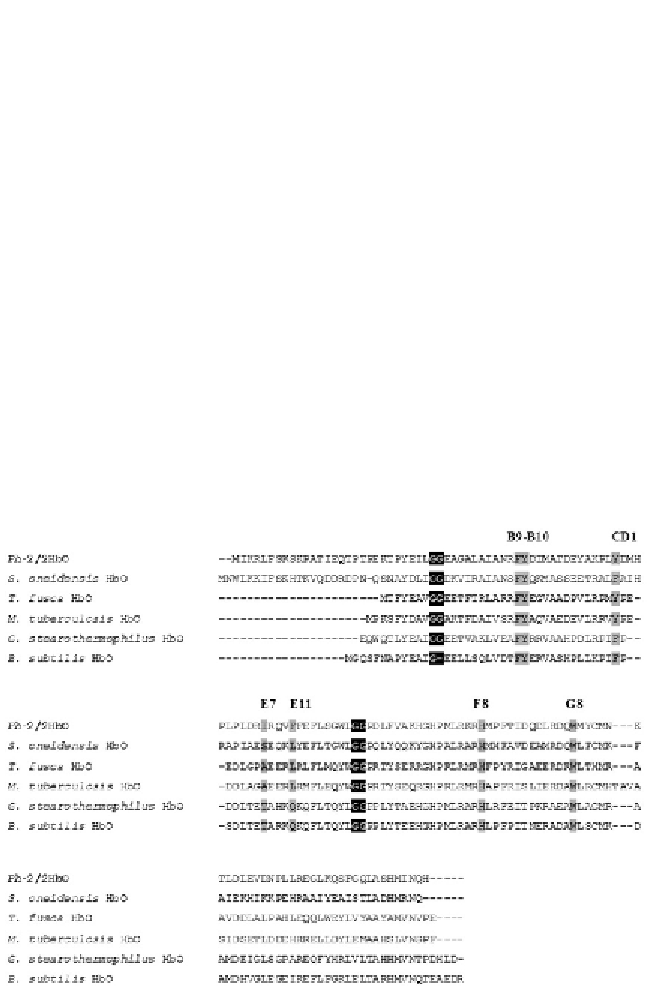
Ph
-2/2HbO displays structural features typical of TrHbII (
Giordano
et al., 2007
). In particular,
Ph
-2/2HbO has Trp at G8, and Tyr at both
CD1 and B10 (
Fig. 8.4
;
Howes et al., 2011
). These three positions are pivotal
for the stabilisation of the haem-boundO
2
in TrHbsII (
Milani et al., 2005
). It
is worth noting that CD1 Phe, that wedges the haem into its pocket, is con-
sidered a conserved residue among globins, unlike members of TrHbsII from
M. tuberculosis
,
Mycobacterium avium
,
M. leprae
,
Mycobacterium smegmatis
,
Strep-
tomyces coelicolor
,
Corynebacterium diphtheriae
and
T. fusca
, which host Tyr
instead (
Table 8.2
;
Bonamore et al., 2005; Milani et al., 2005
).
Figure 8.4 Sequence alignment of some representative TrHbs of group II. Identical
functionally important residues of the distal haem pocket (B9, B10, CD1, E7, E11 and
G8) and the proximal His F8 are highlighted in grey. The Gly-Gly motifs typical of TrHbs
are highlighted in black. Adapted from
Howes et al. (2011).