Biology Reference
In-Depth Information
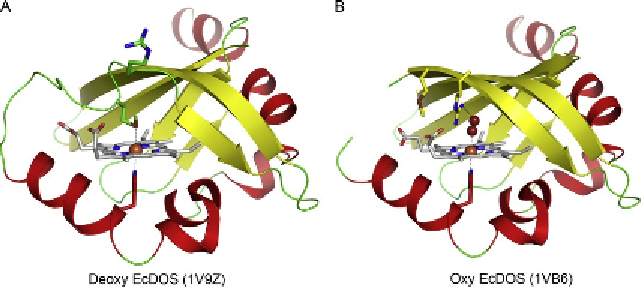
While the FG loop (residues 86-97) is disordered in the ferric
Ec
DosH, it
is significantly rigidified upon reduction of the haem iron (
Kurokawa et al.,
2004
). Electron densities of the FG loop are clearly observed in the ferrous
Ec
Dos, suggesting that the FG loop is rigidified upon reduction of the haem.
As Met95 is located in the FG loop, tethering Met95 to the haem iron will
restrict a flexibility of the FG loop. The distal water molecule (or OH
)
bound to the ferric haem is replaced by Met95 to form a 6-coordinated fer-
rous haem with His77 and Met95 as the axial ligands, which plays an impor-
tant role for rigidifying the FG loop (
Kurokawa et al., 2004; Park
et al., 2004
).
The crystal structure of O
2
-bound
Ec
Dos clearly shows that another
ligand-exchange reaction occurs upon O
2
binding between Met95 and
O
2
, where Met95 is replaced by O
2
to form the O
2
-bound
Ec
Dos.
Met95 tethered to the haem iron is dissociated from it upon O
2
binding,
by which conformational changes in the FG loop are caused. The confor-
mational changes of the FG loop will be involved in the signalling process to
regulate the PDE activity of
Ec
Dos
(
Sasakura, Yoshimura-Suzuki,
Kurokawa, & Shimizu, 2006
).
O
2
binding to the haem also causes rearrangement of hydrogen-bonding
networks around the haem, as observed in FixL. While Arg97 is out of the
distal haem pocket in ferrous
Ec
Dos, the side chain of Arg97 is rotated by
about 180
upon O
2
binding to form a hydrogen bond to the haem-bound
O
2
(
Park et al., 2004
). This hydrogen bond is essential for a stable O
2
binding
to the haem. In Arg97 variants, the stable O
2
-bound
Ec
Dos is not formed
Figure 7.8 The structures of the haem-containing PAS domain of EcDos in (A) the deoxy
(PDB 1V9Z) and (B) oxy (PDB 1VB6) states.