Biology Reference
In-Depth Information
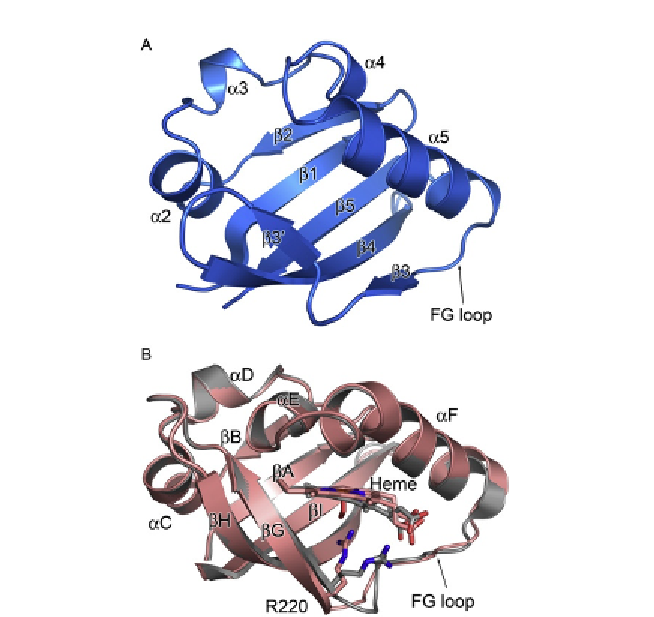
Figure 7.6 Structural comparison of the PAS domain of ThkA and the haem-containing
PAS domain of BjFixL. (A) Structure of the PAS domain of ThkA. (B) Superposition of the
BjFixL PAS domains in the oxy (red) and deoxy (grey) states. Reproduced with permission
from
Yamada et al. (2009)
.
ThkA/TrrA complex, the phosphoacceptor Asp residue in the TrrA faces
the phosphodonor His547 in ThkA (
Yamada et al., 2009
;
Fig. 7.7
). The
interaction between the PAS domain and TrrA is also present in the
ThkA/TrrA complex, in which the
b
3-
b
4 loop and the
a
5 in the PAS
domain in ThkA interact with the
a
4 in TrrA (
Yamada et al., 2009
). The
formation of FixL/FixJ complex changes O
2
-binding affinity of FixL
(
Nakamura et al., 2004
), which may be caused by a similar interaction
between the FixL PAS domain and FixJ.
The position of the CA domain relative to the DHp domain reveals that
the ATP-binding site in the CA domain is far away fromHis547 in the DHp