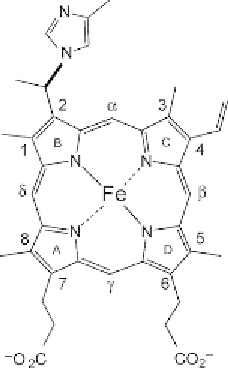
Biology Reference
In-Depth Information
the ferrous state, followed by re-oxidation to the ferric state, however, leads
to a different spectrum, indicating that a modification of the holoprotein
takes place to completion. A combination of NMR spectroscopy and mass
spectrometry identified the modified GlbN (referred to as GlbN-A) as a
covalent adduct involving His117, a histidine in the C-terminal portion
of the H helix (H16;
Vu et al., 2002
): iron reduction caused the addition
of this residue onto the haem 2-vinyl (
Fig. 6.11
). The reaction results in
a pure protein as the small population of alternative
b
haem isomer
eventually becomes depleted by mass action and reaction. Holoprotein
can also be obtained by purification from soluble
E. coli
cell extracts. Once
oxidized to the ferric state, the protein is revealed to be a mixture of both
unmodified and modified protein. The presence of GlbN with covalently
attached haem in the cytoplasm of
E. coli
suggests that the post-translational
modification occurs
in vivo
. Once more, however, it illustrates the risks in
utilizing recombinant material and the exquisite versatility of the haem
group in globins.
The NMR structure of the protein containing a
b
haem can be compared
with the X-ray structure of the protein in the ferric bis-histidine state with
modified haem (
Hoy et al., 2004
). The unreacted His117 is not held in a
rigid position near the vinyl group (
Falzone et al., 2002
) but instead appears
Figure 6.11 The structure of the post-translationally modified haem in Synechocystis
6803 GlbN and Synechococcus 7002 GlbN (
Vu et al., 2002
). A histidine from the
H helix (position H16) adds to the 2-vinyl to form a covalent bond. The modification
is irreversible.