Biology Reference
In-Depth Information
varying ratio is unclear and is likely to remain so until a definite function can
be attributed to the proteins, and the properties of the two isomers are deter-
mined. The fact that the
b
haem is in a dynamic equilibrium in the
holoprotein goes against the rigid view provided by solid-state structures.
From the
in vitro
experimental standpoint, the coexistence of two forms
should not be overlooked because it can cause heterogeneous and compli-
cated responses such as additional kinetic phases in ligand-binding studies.
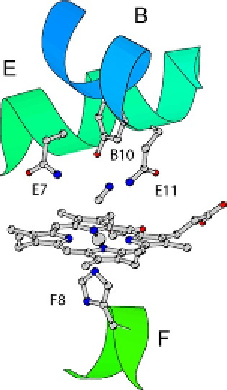
An important feature of the cyanomet structure of CtrHb is the network
of interactions established between the cyanide ligand and distal residues.
Figure 6.9
illustrates the most important of these specific contacts, which
involve Tyr B10, Gln E7 and Gln E11 in a rigid network. Tyr B10 has
the central role as it forms a hydrogen bond with bound cyanide. TrHb1s
contain a nearly conserved Tyr B10 and a glutamine at either E7 or E11,
or at both positions. The network presented by CtrHb is therefore expected
to occur in many other TrHb1s.
Interestingly, the structure of cyanomet CtrHb did not shed much light
on the optical properties mentioned earlier. In the absence of cyanide ligand
and at low pH, water appears to be the sixth ligand to the ferric iron
(
Couture & Guertin, 1996
). The structure of
P. caudatum
Hb, solved in
Figure 6.9 The distal hydrogen bond network in CtrHb as formed in the cyanomet com-
plex (PDB ID 1DLY,
Pesce et al., 2000
) involves Tyr B10, Gln E7 and Gln E11. This network
is expected to form in the majority of TrHb1s.