Biology Reference
In-Depth Information
A second turning point was met in 2000 when Bolognesi and
co-workers published the structures of
P. caudatum
haemoglobin and the
haem domain of
C. eugametos
LI637 haemoglobin, which is depicted in
Fig. 6.2
(
Pesce et al., 2000
). These first two structures of TrHbs anchored
subsequent studies of the entire family. With a three-dimensional frame-
work, progress could be made in the interpretation of the ligand-binding
data being collected in several laboratories on
N. commune
,
C. eugametos
,
and
Synechocystis
sp. PCC 6803 globins. These systematic studies combined
with the structural information allowed for the design of targeted,
hypothesis-driven experiments aimed at delineating the functional roles
of the globins and exploring the physical properties conveyed by the
new fold.
In parallel with the characterization of globins in unicellular photosyn-
thetic organisms, rapid advances were taking place in the field of
haemoglobin at large. New sequences were deposited in databases on what
seemed to be a daily basis, and previously unsuspected members of the super-
family emerged in all kingdoms of life. Globin E (
Kugelstadt, Haberkamp,
Hankeln, & Burmester, 2004
), globin X (
Fuchs, Burmester, & Hankeln,
2006
), globin Y (
Fuchs et al., 2006
), cytoglobin (
Burmester, Ebner,
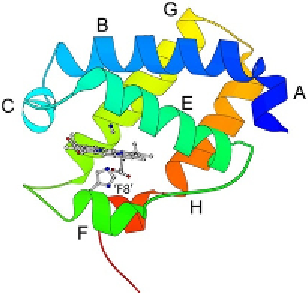
Figure 6.2 Ribbon diagram of ferric C. eugametos LI637 haemoglobin (haem domain
only, CtrHb) in the cyanomet state (PDB ID 1DLY,
Pesce et al., 2000
). The haem, proximal
histidine ('F8'), and cyanide ligand are shown with sticks. Helices are labelled fromA to H.
Compared to
Fig. 6.1
, the A helix is shorter, the D helix is missing, the EF loop is
extended, and the F helix is a reduced to one turn. The topology is a 2/2 orthogonal
sandwich.