Environmental Engineering Reference
In-Depth Information
3.0
2.5
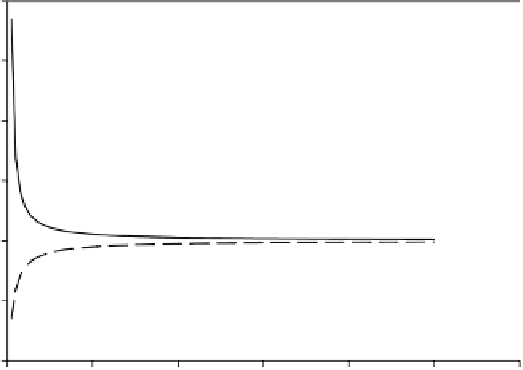
Droplet in air
Meniscus in a capillary
2.0
1.5
1.0
0.5
0.0
0
20
40
60
80
100
120
Radius/
μ
m
FIGURE 3.4
Application of the Kelvin equation for water droplets in air and water confined
in a capillary.
is that in atmospheric chemistry and water chemistry, for very small sizes the effect
of radius on vapor pressure should not be neglected.
The Kelvin effect has been experimentally verified for a number of liquids down
to dimensions as small as 30Å (Israelchvili, 1992). It provides the basic mechanism
for the super-saturation of vapors. The nucleation and formation of clusters from the
vapor phase starts with small nuclei that grow to macroscopic size in stages. The
presence of dust or other foreign particles augments the early stages of nucleation.
In the absence of dust, the enhanced vapor pressure over curved surfaces provides an
energy barrier, and hence the early stage of nucleation will require activation energy.
These and other implications of the Kelvin equation in environmental engineering
will become clear when we discuss the theory of nucleation of atmospheric particles
in Chapter 4.
Now, consider the reverse situation of vapor pressure of liquids confined in small
capillaries or pore spaces such as soils and sediments (Figure 3.5). The situation is
opposite to that of the liquid drops mentioned above. The curvature of the surface is
of opposite sign, and the vapor pressure is
reduced
relative to that at a flat surface.
Therefore we have
exp
.
P
∗
c
i
2
r
σ
V
m
P
i
=
−
(3.46)
RT
Figure 3.6 also shows this relationship for different pore diameters. Liquids that wet
the solid will therefore condense into pores at pressures below the equilibrium vapor
pressure corresponding to a plane surface. This is termed
capillary condensation
and





















Search WWH ::

Custom Search