Environmental Engineering Reference
In-Depth Information
r
+ d
r
r
FIGURE 2.5
Cross-section of an air bubble in water.
and vapor pressure above curved interfaces such as droplets. It also plays a role in
determining the nucleation and growth rates of aerosols in the atmosphere.
2.6.3 S
URFACE
T
HICKNESS AND
G
IBBS
D
IVIDING
S
URFACE
As stated earlier, a surface is not a strict boundary of zero thickness nor is it only a
two-dimensionalarea.Ithasafinitethickness(afewangstroms).Thisposesaproblem
in assigning numerical values to surface properties. Fortunately, Gibbs, the architect
of surface thermodynamics, came up with a simple proposition. It is appropriately
termed the
Gibbs dividing surface
.
Let us consider two phases of volumes
V
I
and
V
II
separated by an
arbitrary
plane
designated
a
. This is strictly a mathematical dividing plane. Gibbs suggested that one
should handle all extensive properties (
E
,
G
,
S
,
H
,
n
, etc.) by ascribing to the bulk
phases those values that would apply if the bulk phases continued uninterrupted up to
the dividing plane. The actual values for the system as a whole and the total values for
the two bulk phases will differ by the so-called
surface excess
or
surface deficiency
assigned to the surface region. For example, the
surface excess
in concentration for
each component in the system is defined as the excess number of moles in the system
over that of the sum of the number of moles in each phase. Hence,
n
i
=
C
i
V
I
−
C
I
i
V
II
.
n
i
−
(2.64)
Γ
i
, mol/m
2
) is defined as the ratio of
n
i
(mol) and
The surface excess concentration (
n
i
/A
(m
2
), that is,
the surface area,
A
.The same equation holds for every other
component in the system. If we now move the plane from
a
to
b
, then the volume of
phase b decreases by
(b
Γ
i
=
σ
σ
a) A
s
and phase a increases by an identical value. Since
the total number of moles in the system is the same, the new surface excess value is
−
new
i
old
i
(C
II
i
C
i
)
Γ
= Γ
+
−
·
(b
−
a)
.
(2.65)


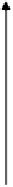







Search WWH ::

Custom Search