Environmental Engineering Reference
In-Depth Information
0.4
RH
RCHO
0.2
NO
2
O
3
NO
PAN
4
8
12
16
20
Time of day/h
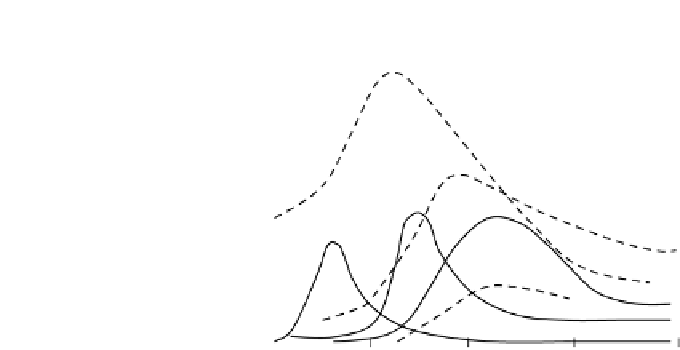
FIGURE 6.50
Diurnal variation in concentrations of various species in a typical urban smog.
In urban areas such as Los Angeles, California, where smog is a common occur-
rence, the concentrations of hydrocarbons (saturated and unsaturated) and aldehydes
are very large. They are produced from automobile emissions. As an example, a
gasoline-powered vehicle exhaust consists of
∼
78% N
2
, 12% CO
2
,5%H
2
O (vapor),
0.06%
NO. The remaining several hundred pptv of oxidized hydrocarbons consist of alde-
hydes, formaldehyde being the dominant fraction. Typical smog composition in Los
AngelesandthediurnalprofileareshowninFigure6.50.Notethepeakconcentrations
of ozone at noontime when smog is severe. The chemistry of smog in urban areas
is incredibly complex due to the presence of a variety of hydrocarbons and aldehy-
des that participate in reactions with ozone and nitrogen oxides. Seinfeld (1986) has
discussed the salient aspects of these reactions, to which the reader is referred to for
more details.
1% unused oxygen,
∼
2% each of CO and H
2
,
∼
0.08% of hydrocarbons, and
∼
6.4 SOIL AND SEDIMENT ENVIRONMENTS
The land surface contributes about 25% of the earth's surface area. Compounds
move between soil and water in the groundwater environment. Sediment is a sink
for contaminants entering water in lakes, rivers, and estuaries. Similarly, soil and air
compartments exchange chemicals. We discuss three examples of transport models,
one for each interface—soil-water, sediment-water, and soil-air.We also discuss soil
remediation concepts that use principles from chemical kinetics.
6.4.1 F&T M
ODELING
We discuss three cases here, namely, groundwater, sediment-water, and soil-air
exchange of chemicals.








Search WWH ::

Custom Search