Environmental Engineering Reference
In-Depth Information
in ppmv or ppbv) by multiplying with 0.0224 m
3
CFC/mol, which is the molar volume
of CFC.
Letusfirstcalculatethevaluesof[CFC],[CFC]
0
,and[CFC]
ss
fortheyear1987when
the Montreal Protocol took effect. In 1987, [CFC]
∼
450 pptv for CFC-12. If we choose
k
−
0.0065 y
−
1
, a production rate for CFC-12 of
Q
s
∼
3
×
10
9
mol/y, and
V
atm
=
3.97
×
10
18
m
3
, we obtain [CFC]
ss
=
2.8 ppbv. If, further, we assume a constant
Q
s
,
then
[CFC]
[CFC]
0
=
6.2
−
5.2e
−
0.0065
t
.
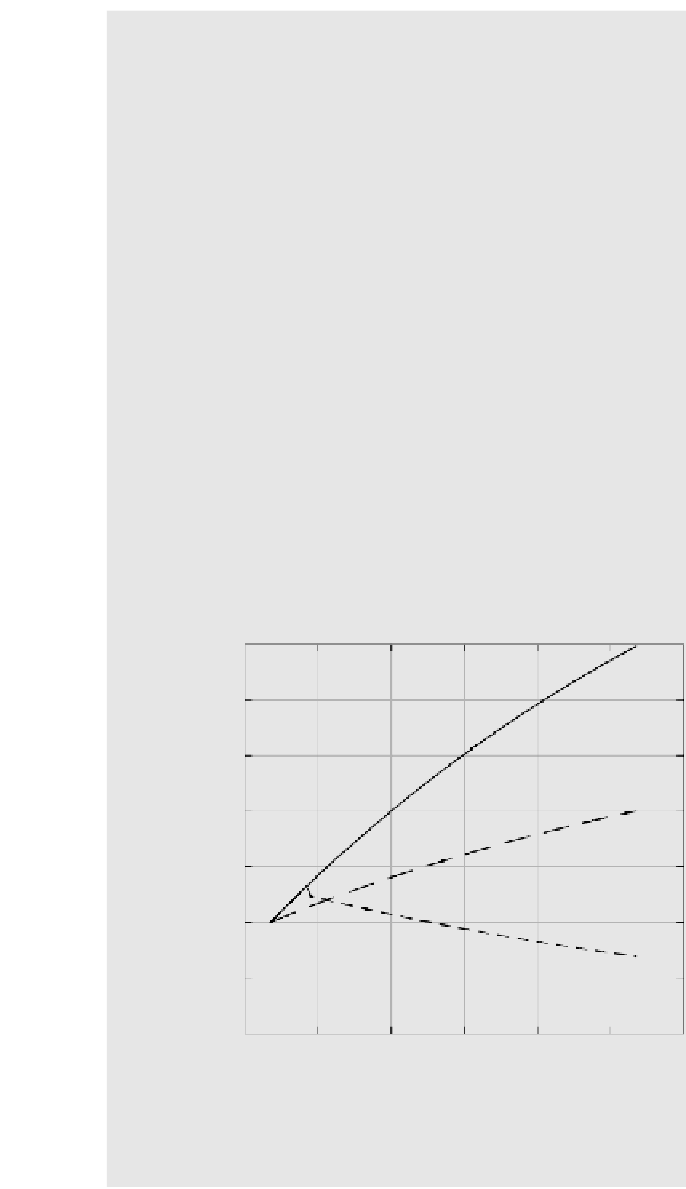
Curve 1 of Figure 6.48 shows the resulting profile. This can be considered to be the
base case
, that is, a consequence of nonimplementation of the Montreal Protocol. If the
Montreal Protocol is to reach a goal of 50% reduction in net CFC emissions, then
Q
s
has to be replaced by 0.5
Q
s
and [CFC]
ss
=
1.4 ppbv. Hence,
[CFC]
[CFC]
0
=
3.1
−
2.1e
−
0.0065
t
.
Curve 2 of Figure 6.48 shows the expected CFC concentration in air in this case. Note
that even under this scenario, the CFC concentration continues to rise in the atmosphere,
albeit at a slower rate. As a consequence, chemical reactions causing the destruction
of stratospheric ozone will continue into the next century. Figure 6.48 also considers
the following scenario: What if the Montreal Protocol had attempted the complete
cessation of CFC production by 1997? The decay of CFC concentration will then be
given by
3.5
3
G
0
2.5
0.5 G
0
2
1.5
G
=
0 in 1997
1
0.5
0
1980
2000
2020
2040
2060
2080
2100
Ye a r
FIGURE 6.48
Atmospheric concentration of CFCs for various scenarios of the
Montreal Protocol.
continued









Search WWH ::

Custom Search