Environmental Engineering Reference
In-Depth Information
77
79
81
83
pH
Pco
2
10
-3
pH
10
-4
8
×
10
-4
6
×
C
T
10
-4
4
×
prese
nt
10
-4
2
×
10
-3
M23
10
-3
M
Ct = [H
2
CO
3
] = [HCO
3
] = [CO
3
]
10
-3
M
22
×
×
24
×
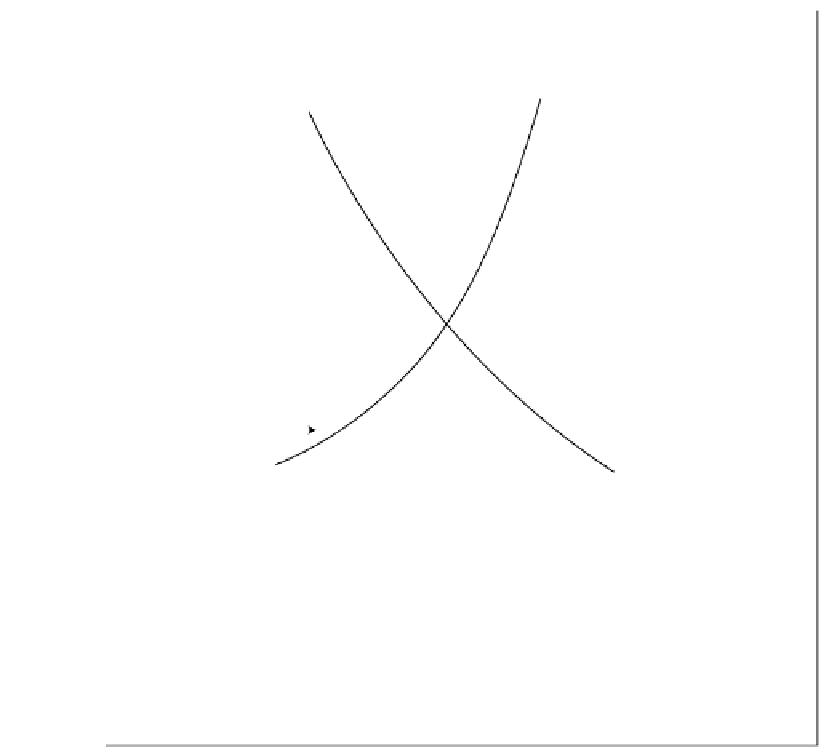
FIGURE 6.44
Effect of oceanic
P
CO
2
upon
C
T
and pH of the ocean water.
The calculations have been made for the following conditions: seawater at 15
◦
C,
P
total
=
1 atm,
[
Alk
]=
constant
=
2.47
×
10
−
3
eq L
−
1
,
[
B
(
OH
)
4
]+[
H
3
BO
3
]=
4.1
×
10
−
4
M, 1
/
H
a
=
4.8
×
10
−
2
mol/L atm.,
K
c1
=
8.8
×
10
−
7
,
K
c2
=
5.6
×
10
−
10
and
K
H3BO3
=
1.6
×
10
−
9
. (Reproduced from Stumm, W. and Morgan, J.J.
1996.
Aquatic Chemistry
, 4th ed., p. 922. NewYork: Wiley. With permission.)
In drawing further conclusions from the above analysis, one should remember
that although the surface ocean is likely in equilibrium with the atmosphere, the
deeper water will reach equilibrium only slowly. This is brought about by mixing
due to upwelling of cold water from deeper layers and subsiding warm water toward
the bottom. This thermal inertia (lag) will likely delay the overall readjustment to
equilibrium for the earth.
Now that we have learned about the increased CO
2
content in the atmosphere, the
next question is how this impacts the temperature of the atmosphere.
Radiative forcing
(
Δ
F)
is a term that represents the amount of heating per m
2
of the surface contributed by a GHG. This is related to the temperature change
contributed by the GHG in the form
Δ
T
= λΔ
F
, where
λ
is a climate sensitivity
parameter (K/W/m
2
). The NRC (1983) report stated that the
Δ
T
associated with CO
2
fluctuations is given by
ln
P
CO
2
P
CO
2
,
Δ
T
= η
(6.167)







Search WWH ::

Custom Search