Environmental Engineering Reference
In-Depth Information
Atmosphere
597 + 165
120
Land
sink
Land
Use
Change
0.2
119.6
2.6
1.6
8.4
GPP
Weathering
Respiration
70.6
70
22.2
20
Fozzil Fuels
3700-244
Ve ge t ar ian
Soil & Debritus
2300 + 101 -140
0.8
0.4
Rivers
Marime Biota
3
Surface Ocean
50
39
Weathering
900 + 18
90.2
1.6
0.2
101
17
Intermediate
& Deep Ocean
37,100 + 100
0.2
Reservoir sizes in GtC
Fluxes and Rates in GtC yr
1
Surface sediment
150
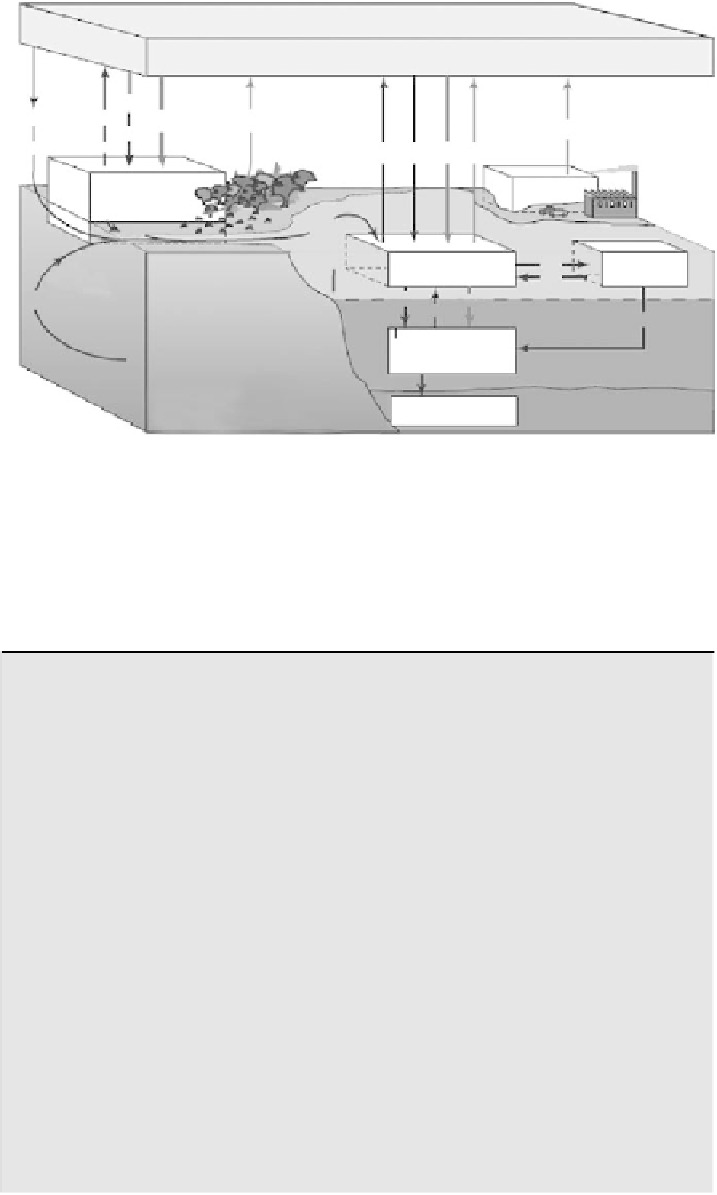
FIGURE 6.42
Global carbon reservoirs and fluxes. Numbers underlined indicate accumula-
tion of CO
2
due to human action. Units are gigatons of carbon for reservoir sizes and gigatons
of carbon per year for fluxes. (Reprinted from Solomon, S., Qin, D., Chen, Z., Marquis, M.,
Averyt, K.B., Tignor, M., and. Miller, H.L. (Eds)
IPCC 2007: Climate Change 2007: The
PhysicalBasis.Contributionofworkinggroup1tothefourthassessmentreportoftheIntergov-
ernmentalPanelonClimateChange,
Cambridge, United Kingdom, and NewYork: Cambridge
University Press, 996 pp.)
E
XAMPLE
6.22 A T
WO
-B
OX
M
ODEL FOR THE
E
FFECT OF
I
NCREASED
A
TMOSPHERIC
CO
2
ON THE
W
ORLD'S
O
CEANS
McIntyre (1978) proposed a model to relate the effects of increased CO
2
emissions on
the world's oceans. Since the ocean is in theoretical equilibrium with the atmosphere,
dissolved CO
2
is quickly converted to HCO
3
. Organic materials that sediment in the
ocean carry HCO
3
downward. Fresh CO
2
from the atmosphere replaces the lost CO
2
in the surface ocean. Plants photosynthesize and remove CO
2
from the air, and are
primarily responsible for keeping the oceans undersaturated with CO
2
. As discussed
earlier, any change in CO
2
concentration is quickly buffered in the ocean. However, if
the rate of change in CO
2
exceeds the rate of establishment of equilibrium, there will
exist a disequilibrium between the ocean and the atmosphere. Both natural processes
(photosynthesis) and anthropogenic (fossil fuel burning) will upset the equilibrium.
How does the ocean then react to this change?
McIntyre (1978) suggested that the terrestrial biosphere is in equilibrium with the
atmosphere, and the marine biosphere is in equilibrium with the surface ocean (up to a
depth of
500 m the ocean is completely mixed in a short period of time). He also made
the assumption that alkalinity in the ocean is only due to carbonates, and that the input of
carbonates from rivers is balanced exactly by its precipitation as CaCO
3
in sediments.
The sediment is, however, not in equilibrium with the atmosphere or the surface ocean.
A two-box model such as represented in Figure 6.43 can then be envisaged.
∼
continued








Search WWH ::

Custom Search