Environmental Engineering Reference
In-Depth Information
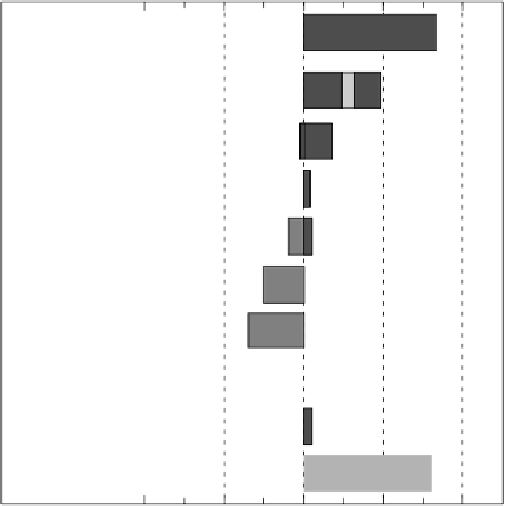
Radiative forcing of climate between 1750 and 2005
Radiative forcing terms
CO
2
Long-lived
greenhouse gases
N
2
O
CH
4
Halocarbons
Tropospheric
Ozone
Stratospheric
(-0.05)
Stratospheric
water vapor
Black carbon
on snow
Land use
Surface albedo
Direct effect
To t a l
Aerosol
Cloud albedo
effect
Linear contrails
(0.01)
Solar irradiance
Total net
human activities
-2
-1
0
1
2
Radiative forcing (Watts per square metre)
FIGURE 6.41
Radiative forcing effects of various GHG gases. (Reprinted from Solomon,
S., Qin, D., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., and. Miller, H.L. (Eds)
IPCC
2007:ClimateChange2007:ThePhysicalBasis.Contributionofworkinggroup1tothefourth
assessment report of the Intergovernmental Panel on Climate Change,
Cambridge, United
Kingdom, and NewYork: Cambridge University Press, 996 pp.)
To start this discussion, we shall first consider the total carbon in solution,
HCO
3
]+[
CO
2
−
3
[
CO
2
]
tot
=[
CO
2
]
aq
+[
]
, and the
Revelle buffer factor
,
R
B
,as
(
[
CO
2
]
tot
/P
CO
2
)(∂P
CO
2
/∂
[
CO
2
]
tot
)
, where [Alk] is the alkalinity of water.
[
Alk
]
OH
−
]−[
HCO
3
]+
CO
2
−
3
H
+
]+[
This is defined (see Chapter 4) as
[
Alk
]=[
2
[
]
.
(P
CO
2
/K
aw
)(
1
H
+
]
Since we have seen in Chapter 4 that
[
CO
2
]
tot
=
+
(K
a1
/
[
)
+
H
+
]
2
))
,wehave
(K
a1
K
a2
/
[
K
a1
H
+
+
.
H
+
−
H
+
+
K
w
P
CO
2
K
aw
2
K
a1
K
a2
H
+
2
[
Alk
]=
(6.162)
Rewriting the equation for the Revelle buffer factor as
∂P
CO
2
/∂
H
+
[CO
2
]
tot
P
CO
2
[
Alk
]
R
B
=
·
∂
[CO
2
]
tot
/∂
H
+
(6.163)
[
Alk
]









Search WWH ::

Custom Search