Environmental Engineering Reference
In-Depth Information
M
Ox
M
k
14
M
O
3
OH
-
k
1
= 140
k
13
M
+
O
-•
⇔
HO
•
M
k
11
M
Ox
×
10
9
k
2
= 1.6
ROO
O
2
k
10
O
-•
O
3
R
•
H
+
×
10
10
k
3
= 5
×
10
5
M
k
4
= 1.4
k
9
HO
•
OH
•
k
12
M
Φ
O
2
FIGURE 6.22
Radical chain reaction mechanism for ozone decomposition in impure water.
(Reprinted from Staehelin, J. and Hoigne, J. 1985.
Environmental Science and Technology
19, 1206-1213. American Chemical Society. With permission.)
Applying the PSSA for O
−•
2
,wehave
OH
−
][
OH
•
]
ss
O
•
2
ss
=
2
k
1
[
O
3
]+
k
9
[
M
][
.
(6.110)
k
2
[
O
3
]
Assuming PSSA for OH
•
, we obtain
O
•
2
k
13
[
M
][
O
3
]+
k
2
[
]
ss
[
O
3
]
OH
•
]
ss
=
[
.
(6.111)
(k
9
+
k
12
)
[
M
]
Solving the above equations, the steady-state concentration of the hydroxyl radical is
2
k
1
[
OH
−
]+
k
13
[
M
]
OH
•
]
ss
=
[
[
O
3
]
.
(6.112)
k
12
[
M
]
Hence we have the following equation:
1
k
9
k
12
.
1
d
[
O
3
]
d
t
=
k
9
k
12
OH
−
]
−
2
k
1
[
+
+
k
13
[
M
]
(6.113)
[
O
3
]



















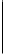













Search WWH ::

Custom Search