Environmental Engineering Reference
In-Depth Information
(b)
(a)
1
1
0.9
0.8
0.8
0.7
0.6
0.6
0.4
0.5
0.4
0.2
0.3
0.2
0
0
2
4
6
8
10
12
0
0.2
0.4
0.6
0.8
1
r
/
R
phi
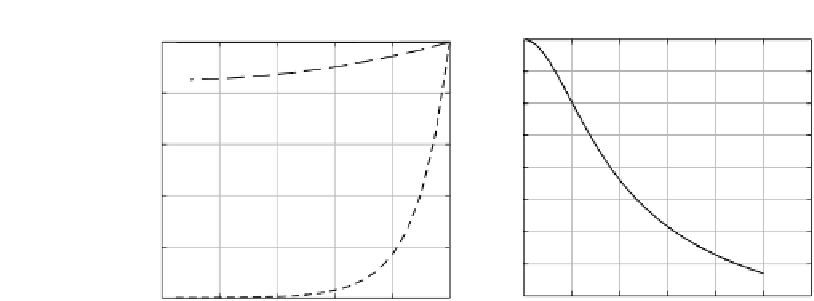
FIGURE 6.13
(a) Variation in pollutant concentration with radial position for various Thiele
modulus. (b) Variation of overall effectiveness factor with Thiele modules.
of the reactant to the interior surface sites is reduced and consequently the process
is diffusion limited within the particle. The overall reaction rate is therefore
r
A
σ
=
ξ
k
1
C
A
σ
if the surface reaction is first order.
Let us now include the external film resistance to mass transfer. This can be intro-
duced to make the problem general and also to evaluate the relative importance of
processes external and internal to the particle. At steady state, the net rate of transfer
of mass to the surface of the particle should equal the net reaction (on exterior surface
and interior surface). Thus,
k
mt
C
A
C
A
σ
A
ext
=
−
r
A
σ
A
int
,
(6.68)
where
A
ext
is the external surface area of the particle and
A
int
is the internal surface
area. Utilizing the expression for
r
A
σ
, we have upon rearranging
k
mt
A
ext
C
A
k
mt
A
ext
+ ξ
C
A
σ
=
k
σ
A
int
.
(6.69)
The steady-state rate of mass transport to the surface is then given by
r
s
mt
=
A
int
C
A
,
r
A
σ
A
int
= ξ
k
A
int
C
A
σ
= ω
k
(6.70)
σ
σ
where
A
ext
))
denotes the change in the effectiveness factor
as the external mass transfer becomes significant. The transfer rate is dependent only
on the bulk phase (air or water) concentration. As
k
ω = ξ
/
1
+
(
ξ
(k
1
A
int
/k
σ
becomes smaller,
ω
decreases
σ
and the external mass transfer resistance becomes important.
Most reactions in environmental systems occur in assemblages of porous particles.
This gives rise to both macropores and micropores within the medium. The diffusion







Search WWH ::

Custom Search