Environmental Engineering Reference
In-Depth Information
6.1.2 N
ONIDEAL
R
EACTORS
The reactor designs we have discussed thus far assumed ideal flow patterns—plug
flow (no mixing) as one extreme and CSTR (complete mixing) as the other.Although
a large number of reactors show ideal behavior, natural environmental reactors fall
somewhere in between the two ideal reactors. Fluid channeling, recycling, or stagna-
tion points in the reactor cause the deviations. There are two models in the chemical
engineering literature that are used to explain nonideal flows in reactors. These are
called
dispersion
and
tanks-in-series
models.
6.1.2.1
Dispersion Model
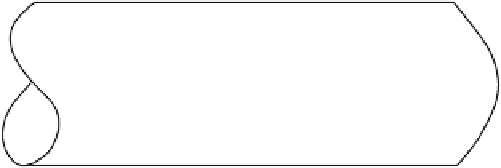
Ideal plug flow involves no intermixing between fluid packets. However, in most
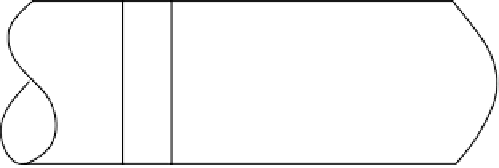
cases molecular and turbulent diffusion can skew the profile as shown in Figure 6.6.
As the disturbances increase, the reactor will approach the characteristic of complete
mixing as in a CSTR. The change in concentration is represented by Fick's equation
for molecular diffusion written as follows:
∂t
=
D
ax
∂
2
C
∂C
∂x
2
,
(6.26)
where
D
ax
(m
2
/s) is called the axial dispersion coefficient. If
D
ax
=
0 we have plug
flow(PFR),andas
D
ax
→∞
theflowiscompletelymixed(CSTR).Tonondimension-
alize the above equation, we express
ut/L
, where
u
represents
the fluid velocity and
L
is the length of the reactor. We have then (Levenspiel, 1999)
ζ =
(ut
+
x)/L
and
θ =
∂
2
C
∂
∂C
∂
θ
=
D
ax
uL
∂C
∂
ζ
−
.
(6.27)
ζ
2
Ideal plug flow
Nonideal plug flow
FIGURE 6.6
Schematic of the plug flow and dispersed plug-flow models.
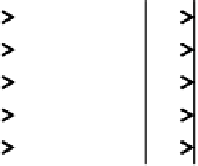

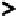






















Search WWH ::

Custom Search