Environmental Engineering Reference
In-Depth Information
in a natural biochemical process. It will also give us information as to how one
can influence a reaction to obtain a desired product in exclusion of other undesired
products. Biochemical reactions can be thermodynamically feasible if the overall free
energy change is negative. However, even if
G
is negative, not all of the reactions
will occur at appreciable rates to be of any use in a living cell unless other criteria
are satisfied. The answer to this is catalysis caused by the enzymes present in all
living organisms.
Enzymes
are a class of proteins that are polymeric chains of amino
acids held together by peptide bonds. Chemical forces arrange these amino acids in
a special three-dimensional pattern. The enzymes are designed to bind a substrate to
a particular site where the conversion to the product species occurs. This active site
is designed to accommodate specific amino acids in a given arrangement for optimal
catalysis.
Enzyme catalysis
is, therefore very specific, but quite fast. In general, there
exists a complementary structure relationship between the active site of the enzyme
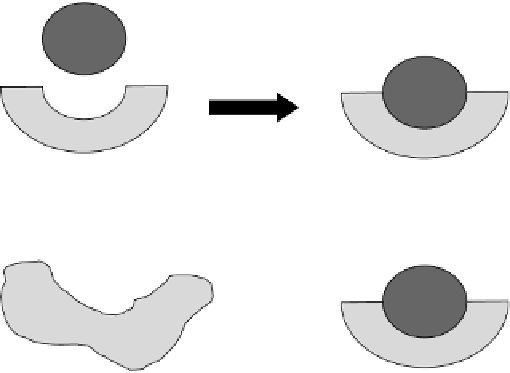
and the molecule upon which the enzyme acts. Two models represent this. The first
one is the
lock-and-key model
, where the substrate and enzyme fit together like a
jigsaw puzzle (Figure 5.18a). In the second model called the
induced fit model
, the
active site wraps around the substrate to adopt the required conformation to bring
about catalysis (Figure 5.18b).
The rate of an enzyme-catalyzed reaction can be obtained by following the
conversion of the substrate into products. The basic mechanism is as follows:
Δ
S
k
1
k
2
−→
E
+
k
−
1
E
−
S
E
+
P,
(5.195)
where E is the enzyme, S the substrate, and P the product. Note that the above mech-
anism is a special case of the general catalysis mechanism described in Section 5.7.
Lock-and-key model
S
S
E
E
S
S
E
E
Induced fit model
FIGURE 5.18
Different models of enzyme-substrate interactions.








Search WWH ::

Custom Search