Environmental Engineering Reference
In-Depth Information
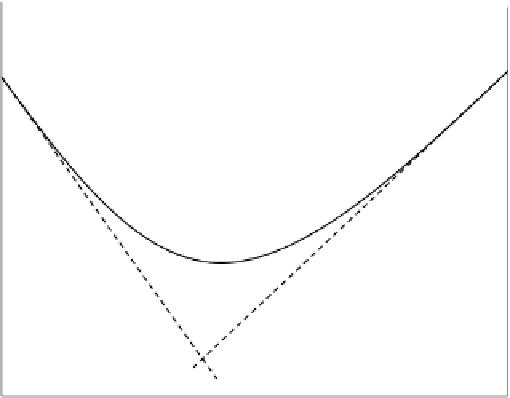
Slope = -1
Slope = +1
Actual curve
log
k
0
I
AN
I
NB
I
AB
pH
FIGURE 5.11
Variation in acid-base hydrolysis rate with pH for organic compounds in the
environment.
a base. Hence, there exist several permutations that should be considered in deriving
rate equations for acid-base catalysis. Moore and Pearson (1981) enumerate nine
such mechanisms. Table 5.4 lists these reaction mechanisms, the rate expressions,
and some examples from environmental engineering.
As noticed in Section 5.4.2, the specific hydrolysis rate constants can be estimated
using an LFER relationship. For example, Wolfe, Zepp, and Paris (1978) showed that
log
k
B
for base-catalyzed hydrolysis of
N
-phenyl carbamates is related to the p
K
a
of
the alcohol group.
An aspect of homogeneous catalysis that we have not considered thus far is the
action of enzymes
(
X
enzyme
)
. This is an important aspect of environmental
bioengineering and is relegated to Chapter 6 where rates of enzyme reactions are
considered.
=
E
XAMPLE
5.12 O
BTAINING
k
o
,
k
A
,
AND
k
B
FROM
R
ATE
D
ATA
The reaction
α
glucose 6
β
glucose is called mutarotation. Acids and bases catalyze it.
At 291 K, the following first-order rate constants were obtained for the process using
acetic acid in an aqueous solution containing 0.02 M sodium acetate.
Acetic acid (mol/L)
0.02
0.105
0.199
k
(min
−
1
)
1.36
×
10
−
4
1.40
×
10
−
4
1.46
×
10
−
4
In the general expression for
k
, both
k
H
and
k
OH
are negligible under these conditions.
k
B
is also negligible under these conditions. Hence,
k
=
k
0
+
k
acid
[acid]. A plot of
k














Search WWH ::

Custom Search