Environmental Engineering Reference
In-Depth Information
5.4.1 A
CTIVATED
C
OMPLEX
T
HEORY
The Arrhenius equation can be interpreted on a molecular basis. The theoretical
foundation is based on the so-called
activated complex theory
(
ACT
).
Weknowthatforthereactantstobeconvertedtoproducts,thereshouldbeageneral
decrease in the total energy of the system. For example, let us consider a bimolecular
gas-phase reaction where an H atom approaches an I
2
molecule to form HI. When H
and I
2
are far apart, the total potential energy is that of the two species H and I
2
.When
H nears I
2
, the I-I bond is stretched and an H-I bond begins to form. A stage will
be reached when the H-I-I complex will be at its maximum potential energy and is
termed the
activated complex
. This is termed the
transition state
. A slight stretching
of the I-I bond at this stage will simultaneously lead to an infinitesimal compression
of the H-I bond and the formation of the H-I molecule with the release of the I atom.
The total potential energy of the HI and I species together will be less than that of the
H and I
2
species that we started with. The progression of the reaction is represented
by a particular position along the reaction path (i.e., intermolecular distance) and is
termed the
reaction coordinate
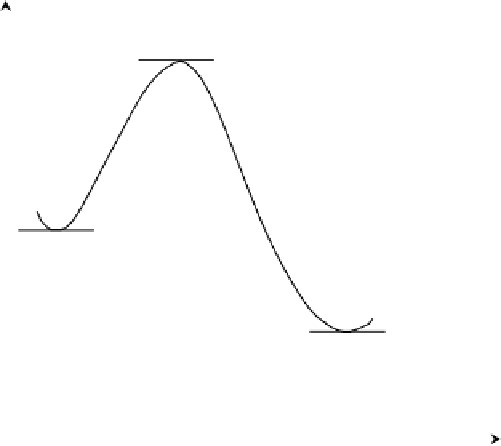
. A plot of potential energy versus reaction coordinate
is called a
potential energy surface
and is shown in Figure 5.10. The transition state
is characterized by such a state of closeness and distortion of reactant configurations
that even a small perturbation will send them downhill toward the products. There is
a distinct possibility that some of the molecules in the activated complex may revert
to the reactants, but those that follow the path to the products will inevitably be in
a different configuration from where they started. In actuality, the potential energy
surface is multidimensional, depending on the number of intermolecular distances
Activated complex
Reactant
Product
Reaction coordinate
FIGURE 5.10
Formation of the activated complex in a reaction.









Search WWH ::

Custom Search