Environmental Engineering Reference
In-Depth Information
5.2 10
-5
5 10
-5
4.8 10
-5
4.6 10
-5
pH = 5.7
pH = 5.0
4.4 10
-5
4.2 10
-5
4 10
-5
5 10
-9
1 10
-8
1.5 10
-8
2 10
-8
2.5 10
-8
0
t
/s
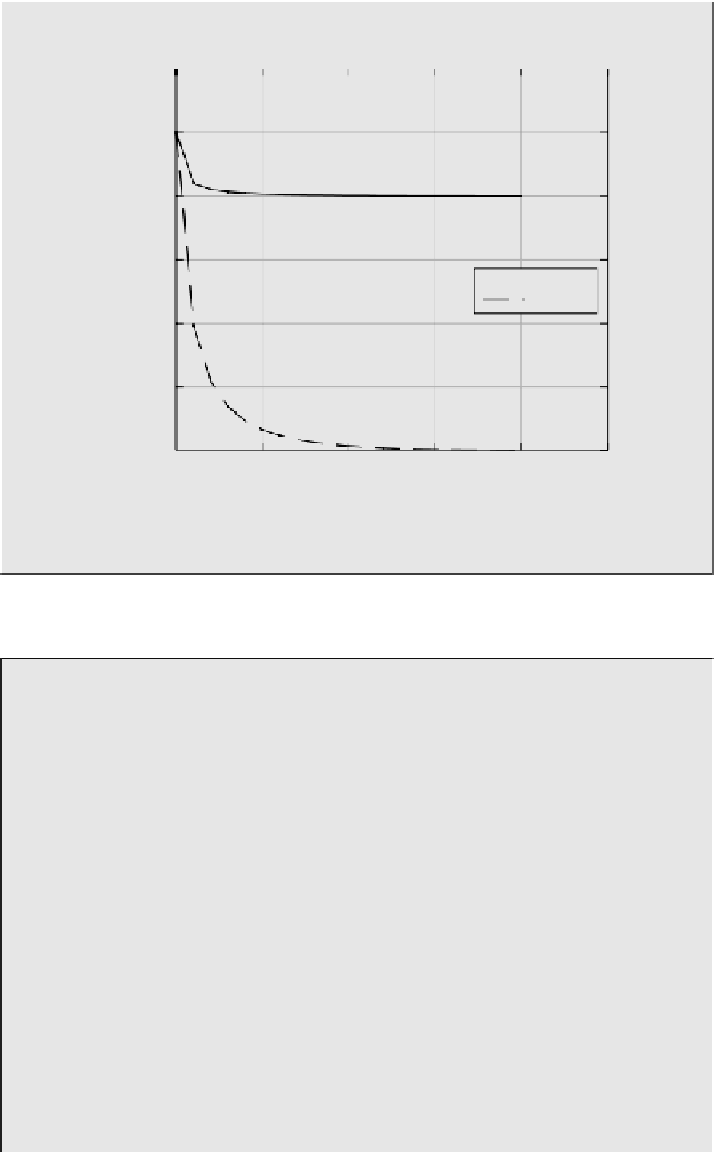
FIGURE 5.8
Kinetics of solution of SO
2
in water at different pH values.
E
XAMPLE
5.7 A
TMOSPHERIC
C
HEMICAL
R
EACTIONS
A large variety of reactions between organic molecules in the atmosphere occur through
mediation by N
2
or O
2
−
, which are the dominant species in ambient air. If A and B
represent two reactants and Z represents either N
2
or O
2
, then the general reaction
scheme consists of the following steps:
A
+
B
k
1
k
−
1
A
−−
B,
A
−−
B
+
Z
k
2
(5.56)
−→
AB
+
Z.
The above is an example of a series reaction with a pre-equilibrium step discussed
earlier. The method of solution is similar.A
−−
B represents an excited state of theAB
species, which is the final product. Typically these excited intermediates are produced
by photo or thermal excitation. This short-lived intermediate transfers its energy to Z
(N
2
or O
2
)
to form the stable AB complex. Thus the overall reaction scheme is A
+
B
+
Z
k
−→
AB
+
Z. Each step in the reaction above is called an
elementary reaction
.
Complex reactions
are composed of many such
elementary reactions
.










Search WWH ::

Custom Search