Environmental Engineering Reference
In-Depth Information
(2
σ
aw
V
w
/
r RT
)
Kelvin equation for pure water droplet
r
*
r
c
r
(
B
1
/
r
) - (
B
2
/
r
3
)
For aqueous solution droplet
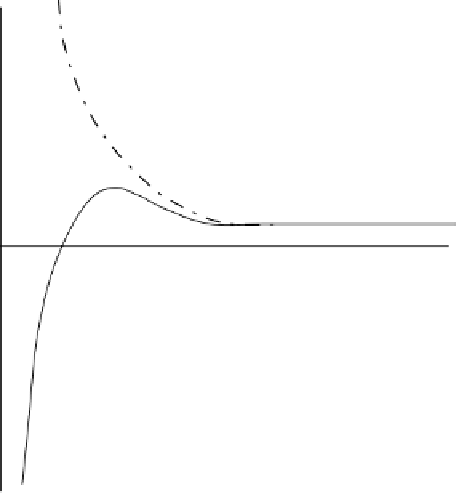
FIGURE 4.6
Kohler curves for pure water droplet and aqueous solution droplet in air. Note
the differences in the shape of the curve when water contains a solute.
and hence the larger particles are activated preferentially. In fact, this is how fog
and cloud droplets that are
−
μ
10
m in diameter are formed from aerosols that are
≈
μ
only
m in diameter. In continental aerosols, the larger particles are generally
efficient condensation points for cloud droplets. The small (Aitken) particles have
negligible contribution toward cloud condensation.
For compounds that do not dissociate (e.g., neutral organics), the value of
n
i
that
appears in the equation for
B
2
is straightforward to estimate. For dissociating species
(e.g.,inorganicsalts),
J
2
shouldincludeboththedissociatedandundissociatedspecies
in solution. If the degree of dissolution is
v
i
(e.g., for HCl the maximum value is 2) and
the initial mass is
m
i
, then
n
i
= ν
i
m
i
/M
i
, where
M
i
is the molecular weight. Generally
dissociation leads to small values of
P
w
/P
w
. If only a portion of the species
i
is soluble
in the aqueous phase (
n
sol
i
0.1
)
and a portion is insoluble (
n
insol
i
)
, then particle growth will
n
inso
i
)V
w
.This makes
the solute effect more pronounced than the previous case when all of
i
was soluble in
the aqueous phase (i.e.,
n
i
in the previous equation is equal to
n
so
i
)
. The experimental
verification of these different cases is reported in the literature (Warneck, 1986).
)(n
sol
i
be retarded somewhat since
B
2
is now defined as
(
3
/
4
π
+
E
XAMPLE
4.15 V
APOR
P
RESSURE ABOVE
A
QUEOUS
D
ROPLETS
Determine the vapor pressure over (i) a 1
μ
m aqueous droplet containing 5
×
10
−
13
g
of NaCl per particle at 298 K, and (ii) a 1
μ
m of pure water droplet.
continued

















Search WWH ::

Custom Search