Environmental Engineering Reference
In-Depth Information
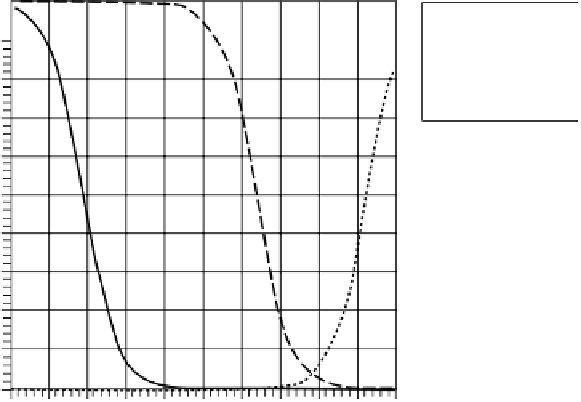
Just as for the SO
2
example, increasing pH decreases the air-water partition con-
stant for the CO
2
case also. The values of the dissociation constants are
K
c1
=
4.28
10
−
11
(Seinfeld and Pandis, 1998).
The effect of pH on the dissolution of ammonia in water is opposite to the ones for
CO
2
and SO
2
.NH
3
dissolves in water to form ammonium hydroxide, which further
dissociates to give NH
4
and OH
−
ions. Thus, the solution is made more alkaline by
the presence of ammonia. As pH increases and the solution becomes more alkaline,
more of ammonia remains as the gaseous species and hence its solubility in water
decreases. Therefore the effective Henry's constant will increase with increasing pH.
The reactions of relevance here are the following:
10
−
7
M and
K
c2
=
×
4.687
×
P
NH
3
[NH
3
·
H
2
O
(
l
)
;
K
aw
=
NH
3
(
g
)
+
H
2
O
(
l
)
NH
3
·
,
(4.27)
H
2
O]
l
NH
4
l
OH
−
l
[NH
3
×
NH
4
(
l
)
OH
−
(
l
)
;
K
a1
=
NH
3
·
H
2
O
(
l
)
+
.
(4.28)
H
2
O]
l
Substituting for
[
OH
−
]=
K
w
/
[
H
+
]
in the second of the above equations, one obtains
the following expression for total ammonia,
1
H
+
]
l
,
P
NH
3
K
a1
K
w
[
[
NH
3
]
T
=
K
aw
·
+
(4.29)
1
SO
2
0.9
CO
2
0.8
NH
3
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
012345
pH
6789 0
FIGURE 4.4
Effect of pH on the air-water partition constant of CO
2
,SO
2
, and NH
3
.












Search WWH ::

Custom Search