Environmental Engineering Reference
In-Depth Information
Atmosphere
Cloud
Dry deposition
(particles)
Volatilization/absorption
Wet deposition
(particles + gas)
Water
Bubble ebullition
Sediment
Bedrock
FIGURE 4.1
Exchange of chemicals between the water and the atmosphere. Equilibrium is
important in many cases such as volatilization-absorption, wet deposition, dry deposition, and
gas bubble transport.
E
XAMPLE
4.1 U
SE OF
A
IR
-W
ATER
P
ARTITION
C
ONSTANT
Water from an oil-field waste pond contains hydrogen sulfide at a concentration of
500 mg/L at an ambient temperature of 298 K. What will be the maximum equilibrium
concentration of H
2
S above the solution?
At 298 K,
K
aw
for H
2
S is 0.41 (Appendix 1).
C
i
w
=
500 mg/L
=
0.5
/
34
=
0.0147 mol/L. Hence
C
i
a
=
(
0.41
)(
0.0147
)
=
0.006 mol/L. Partial pressure in air,
P
i
=
C
i
a
RT
=
0.147 atm.
Many
waste treatment separation processes
also involve air-water exchange of
chemicals (Figure 4.2). Examples include wastewater aeration ponds and surface
impoundments in which surface and submerged aerators are used to transfer oxygen
to the water, and to remove volatile organic and inorganic compounds from water
using flotation. Volatile organics are removed using a tower where air and water
are passed countercurrent to one another through a packed bed. The operations are
nonequilibrium processes, but where, the tendency of the system to attain equilibrium
provides the driving force for separation.
The transfer of material (say
i)
from one phase to the other (e.g., water to air) is
due to a gradient in concentration of
i
between the phases (Figure 4.3). This flow of
material
i
from water to air is termed
flux
and is defined as the moles of
i
passing a
unit area of interface per unit time. The equation for flux is
K
w
C
i
w
−
C
i
w
,
N
i
=
(4.3)



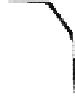






















Search WWH ::

Custom Search