Environmental Engineering Reference
In-Depth Information
satisfactorily at low
C
i
values, whereas significant deviations are observed at high
C
i
values. The Langmuir isotherm severely under-predicts adsorption at high
C
i
values,
whereas the Freundlich isotherm severely over-predicts the adsorption in the same
region.Thus neither of the isotherms can be used over the entire region of concentrations
(a)
4.00E + 4
(b)
-8
-8.1
-8.2
-8.3
-8.4
-8.5
-8.6
-8.7
-8.8
-8.9
-9
3.50E + 4
3.00E + 4
2.50E + 4
2.00E + 4
1.50E + 4
1.00E + 4
5.00E + 3
0.00E + 0
-9
-8.5
-8
-7.5
-7
-6.5
-6
log
C
i
1/
C
i
(L/mol)
FIGURE 3.13
Langmuir (a) and Freundlich (b) isotherm plots for the adsorption of
an insecticide (chlordane) on GAC.
0.00030
0.00025
0.00020
0.00015
0.00010
Langmuir
Freundlich
Experimental
0.00005
0.00000
0.0
5.0e-8
1.0e-7
1.5e-7
2.0e-7
2.5e-7
3.0e-7
3.5e-7
C
i
/mol
∙
L
-1
FIGURE 3.14
Adsorption isotherms for chlordane on GAC.






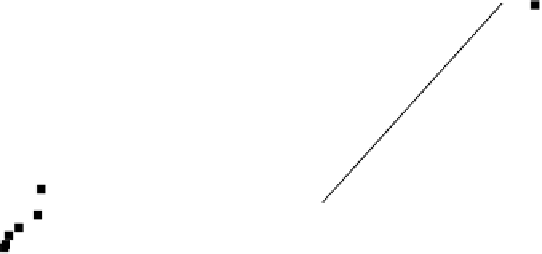









































Search WWH ::

Custom Search