Environmental Engineering Reference
In-Depth Information
Noting further the dilute solution approximation for
x
i
,
we have
θ
i
− θ
i
=
K
Lang
·
C
i
.
(3.80)
1
The adsorption isotherm given above is called
the Langmuir adsorption isotherm
.It
is particularly useful in a number of situations to represent the adsorption data. In
the case of the solid-gas interface, the concentration term is replaced by the partial
pressure of solute
i
in the gas phase. In fact, Langmuir first suggested this equation
to represent gas-phase adsorption data in catalysis.
It is important that one should know how to use experimental data to obtain appro-
priate adsorption isotherm parameters. In the case of a linear adsorption isotherm, it is
obvious that a plot of
Γ
i
versus
C
i
should be linear with a slope of
K
Γ
l
. However, when
a plot of the same is made for a Langmuir isotherm, a linear behavior is displayed
at low
C
i
and approaches an asymptotic value at high
C
i
values. The linear region is
characterized by
m
i
Γ
I
=
K
Lang
C
i
, and .we have
Γ
i
= Γ
(Figure 3.12). In practice, for
the Langmuir isotherm, one plots 1
/
Γ
i
versus 1
/C
i
, the slope of which gives an inter-
i
)
−
1
. If one takes the intercept over the
slope value, one obtains directly
K
Lang
. Just the mere fact that a given set of data fits
the Langmuir plot does not necessarily mean that the adsorption mechanism follows
that of the Langmuir isotherm. On the contrary, other mechanisms such as surface
complex formation or precipitation may also lead to similar plots.
An empirical relationship that represents any set of data on adsorption at low con-
centrations is called the
Freundlich adsorption isotherm
. Apart from its universality
in data representation, it was thought to have little theoretical value for a long
time. Recently, it has been shown that it can be derived theoretically by considering
the heterogeneous nature of adsorption sites (Adamson, 1990; Sposito, 1984). The
Freundlich isotherm is expressed as follows:
m
i
)
−
1
and an intercept of (
m
cept of (
K
Lang
Γ
Γ
K
Freun
(C
i
)
1
/n
,
Γ
i
=
(3.81)
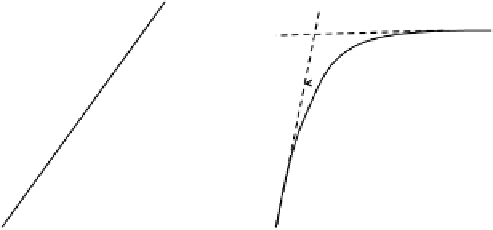
Linear isotherm
Langmuir isotherm
Γ
i
m
Slope
K
Lang
Slope
K
H
C
iw
/mol
∙
cm
-3
C
iw
/mol
∙
cm
-3
FIGURE 3.12
Schematic of linear and Langmuir adsorption isotherms.










Search WWH ::

Custom Search