Environmental Engineering Reference
In-Depth Information
14.4.3 S
ourceS
and
S
InkS
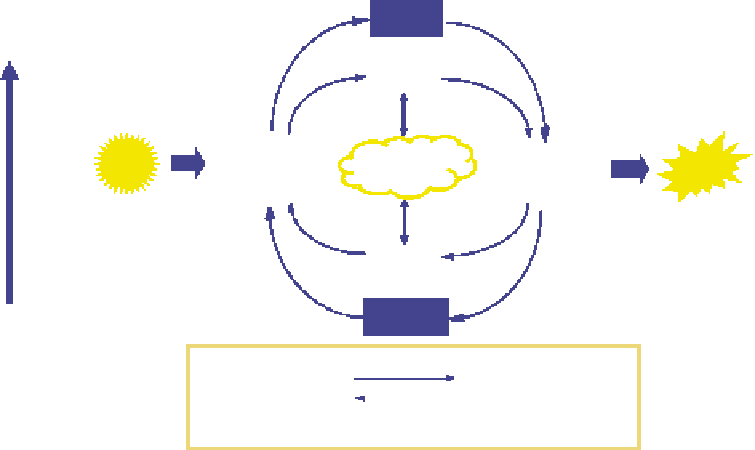
The factors affecting DO in lakes and reservoirs include the production and decomposition of
organic matter, as illustrated by Figure 14.8. One source (or sink) of oxygen is exchange with the
atmosphere. Unlike rivers that are dominated by stream turbulence, most of the exchange in lakes
is due to wind and waves. The exchange is based on the rate of turbulence and the difference in the
partial pressures of the oxygen (or the differences between the oxygen concentration and the satura-
tion concentration). Depending on the degree of vertical mixing, the exchange may contribute to an
oxygenated surface layer (epilimnion) but not to deeper waters. At times of high productivity, the
surface waters of some lakes may become supersaturated, resulting in the venting of oxygen to the
atmosphere. Oxygen may also be artiicially injected into some lakes as a management or restora-
tion practice, which will be discussed in later chapters.
Organic material added to lakes and reservoirs may be derived from external sources (point
and nonpoint loads, such as tributaries, waste discharges, and runoff) or internal sources. Lakes
and reservoirs often differ from streams and rivers, particularly low-order systems, in that much
of the organic material is autochthonous rather than allochthonous. While generalizations are
often problematic, the relative importance of the littoral components of autochthonous produc-
tion increases greatly and changes markedly from nutrient-limited to nutrient-rich lakes (Wetzel
20 01).
The stoichiometric relationship between production and respiration or the decomposition of
organic matter is commonly based on some assumed representative reaction, such as shown in
Equation 14.9 assuming that ammonium is a substrate (Chapra et al. 2008):
+
2
−
+
106CO HHPO
+
16
+
+
108
HO CHONP OH
+
107
+
14
(14.9)
2
4
4
2
106
263
110
16 1
2
where the forward reaction represents production and the backward reaction represents respiration.
If that (Equation 14.9) were the case, then for each gram of carbon degraded or respired, 2.67 g of
oxygen (as O
2
) would be consumed as computed by
Organic
matter
High energy
O
2
Production
Autotrophs
(plants)
Decomposition
Heterotrophs
(bacteria/animals)
Solar
energy
Atmosphere
Chemical
energy
CO
2
Inorganic
nutrients
Low energy
Photosynthesis
6CO
2
+
6H
2
O
C
6
H
12
O
6
+
6O
2
Respiration
Carbon
dioxide
Water
Sugar
Oxygen
FIGURE 14.8
Natural cycle of organic production and decomposition. (Modiied from Chapra, S.C.,
Surface
Water Quality Modeling
, McGraw-Hill, New York, 1997.)
Search WWH ::

Custom Search