Geology Reference
In-Depth Information
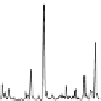
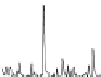
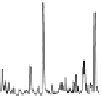
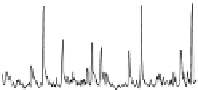
Fig. 1.1
Total ion chromatogram from Py-GC-MS of modern
Metasequoia glyptostroboides
after
lipid extraction (sample 1), and at various stages of natural decay (samples 4-6). Ps: polysaccha-
ride derivative; P: phenol; Bn: alkyl phenol; Pn: alkyl phenols, where n denotes number of carbons
in the alkyl component; Vp: vinyl phenol, G denotes guaiacyl lignin units (
G1
2- methoxy phenol,
G2
2-methoxy-4-methyl phenol,
G3
4-ethenyl-2-methoxyphenol;
G5
3-allyl-6-methoxyphenol);
Lv
Levoglucosan;
C
16:2
FA
C
16
diunsaturated fatty acid,
C16FA
saturated fatty acid
Figure
1.2
highlights the molecular species of interest in this study: the mass
chromatograms showing the distribution of the pyrolysis products of vinyl phenol vs.
lignin (Fig.
1.2a
) and vinyl phenol vs. cellulose (Fig.
1.2b
), respectively. Clearly, the
guaiacyl lignin units in sample 1 (undecayed) decrease progressively in abundance to
vinyl phenol (through sample 4-6, Fig.
1.2a
); a trend observed in levoglucosan derived
from cellulose when compared to vinyl phenol (Fig.
1.2b
). Vinyl phenol may be pro-
duced by thermal breakdown of the
p
-hydroxyphenol unit in certain types of lignin as
a part of woody tissue, but in leaves it is mainly related to
p
-coumaric acid. It is pres-
ent both as ester- and ether-linked units in woody tissues (as part of lignin), and also
as part of decay-resistant cuticle (Tegelaar et al.
1989
). Vinyl phenol (4-ethenyl phe-
nol) is produced, along with C
16
unsaturated fatty acids as the primary thermal break-
down product during pyrolysis of cutin (Tegelaar et al.
1989
) and isolated cuticles
(Mösle et al.
1998
) and is derived from
p
-coumaric acid as part of decay-resistant
cuticle (Tegelaar et al.
1989
). For these reasons, changes in the chemical composition
of the leaves have been determined by the abundance of specifi c moieties relative to
that of vinyl phenol. Vinyl phenol has been used as a marker for cutin and cuticle in






























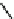


















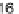


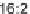









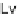





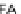
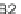








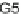














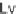





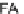



















Search WWH ::

Custom Search