Geology Reference
In-Depth Information
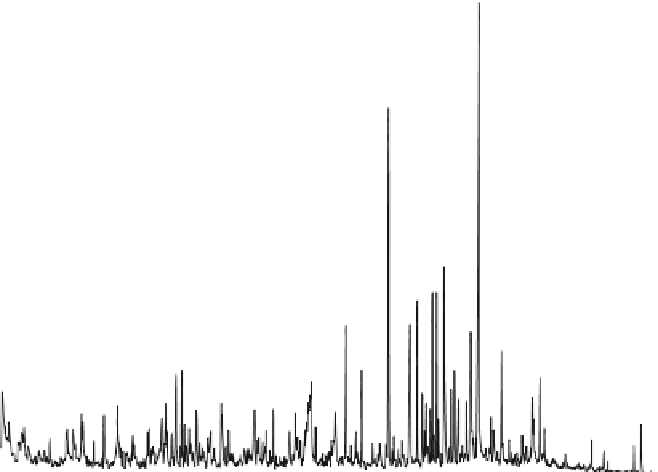
Modern Bacteria (
Oscillatoria
sp
.
)
C
16
FA
*
C
16
uFA
C
14
FA
Pr
I
Pr
C
18
FA
Ps
C
16
FA
Pr
Pr
Ps
Ps
Ps
Ps
*
Pr
Pr
Ps
*
Retention time
Fig. 10.1
Gas chromatographic analysis of modern
Oscillatoria
after solvent extraction,
thermodesorption and pyrolysis.
Pr
: Proteins,
Ps
: Polysaccharides, C
16
FA: C
16
Fatty acid, C
16
uFA:
C
16
unsaturated fatty acid, C
18
FA: C
18
fatty acid, C
14
FA: C
14
fatty acid,
B
: benzene derivative,
P
: Phenol derivative,
I
: Isoprenoid, *: phthalate contaminant
1993
). Alkanes are abundant in the solvent extracted fraction from sediments and
bacteria but homologous alkane-alkene doublets, as seen here after pyrolysis, repre-
sent macromolecularly-bound aliphatic compounds and do not represent presence
of compounds from the soluble fraction (Larter and Horsfi eld
1993
). As modern
bacteria lack any resistant biopolymers, including resistant aliphatic biopolymers
(Allard et al.
1997
), the aliphatic residue-containing macromolecule we observe is
a product of simulated thermal metamorphism. Interestingly, fatty acid molecules
up to C
18
are observed along with alkyl amides, the latter formed by the reaction of
fatty acids with nitrogen containing molecules such as proteins (Gupta et al.
2006
).
Alkane and alkenes are highly soluble in solvent used in this study and also volatile,
thus their abundance in the pyrolysate of the insoluble residue indicate the presence
of covalently bound functionalized lipids into the thermally generated macromole-
cule. Experiments at 260 °C resulted in a similar macromolecular composition as
was obtained at 350 °C (Fig.
10.2
). Analysis of the heated mixture of C
16
and C
18
fatty acid after thermodesorption yielded alkane-alkene peaks up to C
18
along with
C
16
and C
18
fatty acids (Fig.
10.3
), indicating that the lipids were a source of the





Search WWH ::

Custom Search