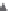Environmental Engineering Reference
In-Depth Information
750
700
120
8 × 10
-5
mol/L
650
Al
13
Al
13agg
.2.6
Al
13agg
.2.7
Al
13agg
.2.8
600
100
550
500
80
450
400
350
60
300
10
-5
mol/L
250
40
200
150
Al
13
Al
13agg
.2.6
Al
13agg
.2.7
Al
13agg
.2.8
20
100
50
0
0
-50
0
200
400
600
800
1000
0
200
400
600
800
1000
Time
Time
FIGURE 38.3
PSD effect of coagulation with nano-IPF: growth of loc.
Production of high-Al
13
nano-IPF
Gibbsite
Al(OH)
3
gel
Ca(AlO
2
)
2
HCl
T
º
C, P
Spray
dried
Powder
product
Al
13
> 45%
Al
13
> 80%
Lab. microtitration
Nano-tech
material
Electrochemical
Al
13
> 80%
Al
13
> 80%
Membrane technology
Al
13
> 90%
Chem. separation
FIGURE 38.4
Schematic description of the industrialization of nano-IPF. SKLEAC, State Key Laboratory of Electroanalytical
Chemistry.
The current industrial production through high-temperature/high-pressure dissolution-
second alkaliication process can yield only <45% Al
13
. On the other hand, in laboratory
microtitration, it is easy to reach as high as 80% Al
13
. For an industrial-scale production,
the possible ways to reach high yields include electrochemical preparation, membrane
technology, and chemical separation and puriication. Figure 38.5 shows a demo-plant
established in Beijing, China, using the electrochemical preparation method.
The separation and puriication of Al
13
in PACl with various concentrations (0.05-0.5
mol/L) using the ultrailtration (UF) method have also been investigated (Huang et al.,
2006b). The relation between the species distribution data obtained by the ferron assay and
the interception data of UF membranes with different molecular weight cutoffs (MWCOs),
in which the concentration factor is neglected, is presented in Figure 38.6.
It can be clearly discovered that if the content of Al
b
rises in PACl, the fraction with
an MWCO >6 kilodaltons (kD) does not augment obviously, the 3-6 kD MWCO fraction