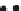Environmental Engineering Reference
In-Depth Information
1.0
0.8
0.6
Fluoride inlet concentration
(mg/l)
0.4
5
15
30
0.2
0
0
100
200
Time (min)
300
400
FIGURE 36.10
Breakthrough data for varying inlet luoride concentration (
W
Al-CNF
= 3 g,
Q
= 0.01 l/min,
T
= 303 K). (From
A. Gupta et al.,
I&EC Res
., 48, 9697, 2009.)
the equilibrium ion loading shown in Figure 36.9 for all three concentrations, within the
experimental and calculation errors, which suggests that the Al-CNF-based adsorbents
are suitable for developing water puriiers in deluoridation applications.
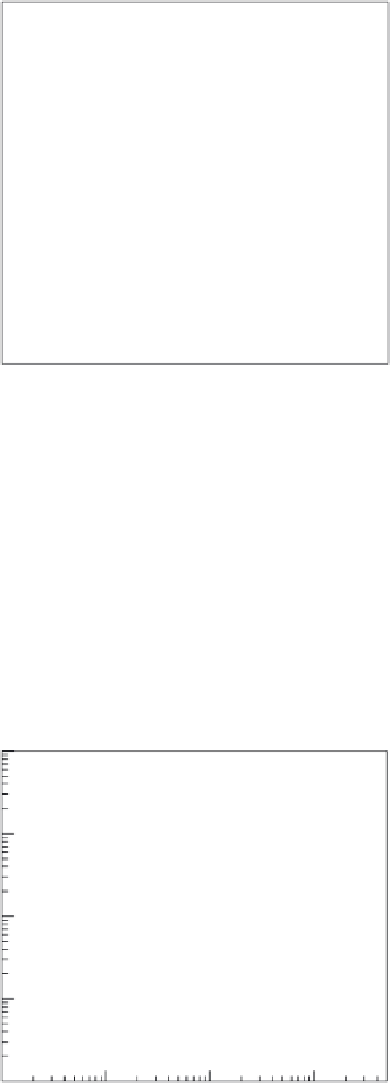
Fe-CNFs have also exhibited signiicant adsorption capacity for As(V). Figure 36.11
describes the adsorption isotherms for As(V) on different ACF/CNF-based adsor-
bents. From the isotherms shown in the igure, the equilibrium loading of arsenic may
be observed to be much higher on Fe-CNF (micro-/nanoweb) compared with the other
100
Fe-CNF
Fe-ACF
ACF
10
Freundlich equation
q = K
×
C
n
1
0.1
0.0
0.01
0.1
Aqueous-phase arsenic concentration (mg/l)
1
10
FIGURE 36.11
Comparative performance of Fe-CNF, Fe-ACF, and ACF for arsenic removal. Solid lines show Freundlich iso-
therms,
q
=
K
×
C
n
, where
K
= 1.5, 0.36, 0.2, and
n
= 0.59, 0.56, 0.52 for Fe-CNF, Fe-ACF, and ACF, respectively.
(From A. Gupta et al.,
I&EC Res.
, 49, 7074, 2010.)