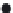Environmental Engineering Reference
In-Depth Information
0
1
2
3
4
5
6
7
60
60
50
50
40
40
Ca
+
Na
+
Mg
+
Zn
+
K
+
30
30
20
20
10
10
0
0
0
1
2
3
4
5
6
7
Time (h)
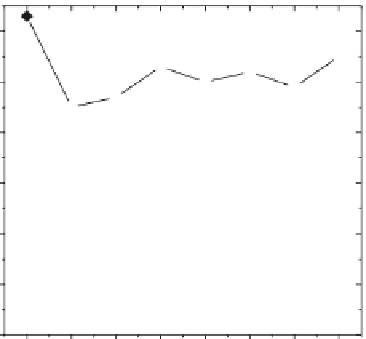
FIGURE 30.5
Performance of the different cations in the ion-exchange process with ZZ.
determined at the end of the experiment. The results (Figure 30.6) showed that zinc content
increased from a ratio of 1:1 to 2:1; however, the longer the exposure, the lower the zinc
content in the drinking water. The inluence related to the length of the exposure indicates
that the initial ion exchange of Zn
2+
in clinoptilolite by Ca
2+
ions was reversed. Thus, the
equilibrium of clinoptilolite (ZZ) with Zn
2+
as an extra-framework cation is better than
with Ca
2+
ions.
The amount of Zn released by the clinoptilolite structure to the drinking water was
<5 mg/L, which meets the WHO
Guidelines for Drinking Water Quality
[13]. This zinc con-
tent is enough for a bactericidal effect on pathogenic bacteria, as will be shown later on in
this chapter.
The kinetics of the exchange reaction of zinc ions in ZZ by calcium ions present in
drinking water was studied at different water low rates in the ion-exchange columns.
0
2
4
6
8
10
6
6
1st hr
2nd hr
3rd hr
4th hr
5
5
4
4
3
3
2
2
1
1
0
0
0
2
4
6
8
10
Volume of water/mass of ZZ
FIGURE 30.6
Zn
2+
content in drinking water as a function of volume of water/mass of ZZ.