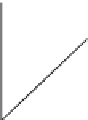Environmental Engineering Reference
In-Depth Information
reuse with the exception of sodium levels and TDS (approximately 1110-1550 mg/l, where
<1000 mg/l is desired), which vary based on rainfall and other factors. EDR is a good tech-
nology for delivering consistent product water quality in applications where feedwater
quality is good but variable. The plant removes 55% of the salts, operates at an 85% product
recovery rate, and produces 2.2 MGD of product water [41].
Projections by Siemens predict that in 2030 and beyond, Siemens will provide nearly 75%
of urban water demand in Australia through continuous electrodeionization technologies
reaching an estimated population of 25 million people at energy consumption levels of
1 MWh/Ml [42]. Electrodialysis- and EDR-based technologies are also being investigated and
employed by GE in many of their water desalination plants. The promise of low energy usage
as shown has also encouraged use of other electrical methods, such as capacitive deioniza-
tion, which are being used by companies (e.g., PROINGESA) in Europe [43].
27.2.3.6 Challenges with Membrane Processes
Since RO desalination is the most commonly new installed desalination plant type world-
wide, it is important to discuss some of the challenges that RO and other membrane-
based technologies face. As with any technology, challenges and limitations to membrane
separations must be accounted for in water treatment design and implementation. Major
impedances to membranes separations include concentration polarization, fouling, and
precipitation/scaling, which dictate, partially and sometimes fully, product recovery pos-
sible, maintenance protocol and procedures, and membrane lifetime. Concentration polar-
ization occurs when, as membrane separation processes develop, concentration gradients
form at the surface as the rejected feed components build up near the membrane on the
reject side [44], which forms a higher concentration layer at a membrane surface as seen
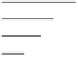
schematically in Figure 27.9. This, in turn, leads to an increased local osmotic pressure at
the membrane surface, which causes a decrease in the effective driving pressure [45,46],
leading to an eventual drop in permeate lux. As polarization persists and fouling contin-
ues to develop, particles adhered to the membrane surface can cause scaling, which occurs
when solid phase precipitates out of the solution, or bioilm formation. In addition to lux
impediment, bioilms can act as a source of permeate contamination. This denser, more
impeding layer can be termed a cake or gel layer, as shown in Figure 27.10 [44].
Concentration polarization (
N
F
<
N
Fc
)
y
Concentration polarization layer
Particle
suspension
x
Membrane
Permeate flux
FIGURE 27.9
Concentration boundary layer formed by concentration polarization on a membrane surface. The igure shows
the development of the concentration polarization boundary layer for cross-low over the membrane, with the
permeate passing through the membrane as shown. This concentration boundary layer causes lux impedi-
ment due to the increase in local concentration of species. (From Chen, J.C., Q. Li, and M. Elimelech,
Advances in
Colloid and Interface Science
, 107, 83, 2004.)