Environmental Engineering Reference
In-Depth Information
(a) Cryogelation
Freezing
Polymerization
Monomer
solution
Frozen
solution
Macroporous hydrogel
(b) Use of porogen
Polymerization
Porogen
gas
formation
Monomer
solution
Macroporous hydrogel
SCHEME 2.6
Macropore formation mechanism by templating with ice crystals in cryogelation (a) or by
in situ
gas formation (b).
(b)
(a)
HFW
43.5 µm
Dot
SED
10 µm
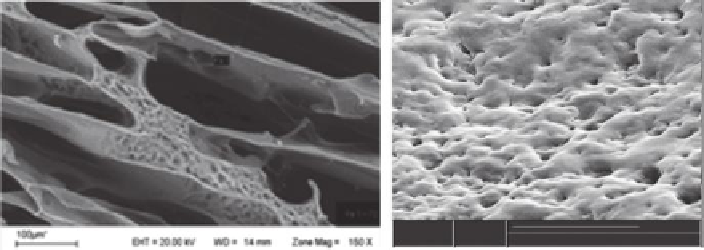
FIGURE 2.9
Scanning electron micrographs of macroporous hydrogels made by cryogelation (a) and using oxalate ion
(which produces CO
2
gas by oxidation inside the gel) as porogen (b).
small spherical pores produced by the formation of gas bubbles inside the polymerization
solution (Figure 2.9b).
Besides functionalization with nanoparticles, nanocomposites could be produced by
in
situ
formation of another polymer inside the hydrogel. We produced different hydrogels
(PAAm, PNIPAM, PAMPS) containing conducting polymers (PANI, PPy, PNMANI) by
oxidative polymerization of the conductive polymer's monomer inside the hydrogel.
2.2.3 Nanocomposites
2.2.3.1 Conductive Polymer Filling the Nanopores of a Hydrogel
Conducting polymers could be used to remove pollutants by metal ion reduction [123],
or nucleophilic addition of the pollutant to the polymer [124]. A simple way to produce