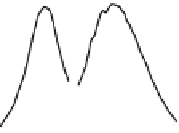Environmental Engineering Reference
In-Depth Information
(a)
(b)
726
590
λ
ex
λ
em
28k
0.15
21k
0.10
14k
b
500
600 700 800
Wavelength (nm)
7k
0.05
a
0
0.00
400
600
800
1000
20
40
60
80
Wavelength (nm)
2θ (degree)
FIGURE 26.6
(a) UV/Vis absorption spectrum of Ag
9
(MSA)
7
QCs. Inset is the PL spectra of Ag
9
(MSA)
7
cluster emitting at
726 nm when excited at 590 nm. (b) XRD patterns of as-prepared AgCl (trace
a
) and reaction product (trace
b
)
of cluster and CCl
4
in the presence of IPA. (Adapted from Bootharaju, M.S. et al.,
J. Mater. Chem. A.
, 1, 611, 2013.
Copyright with permission from The Royal Society of Chemistry.)
been understood to some extent. It was found that the solvent used (2-propanol) played
an important role in the degradation of halocarbon. The electrons released in oxidation
of IPA in the presence of metal clusters/NPs were abstracted by chlorocarbon to yield
chloride ions. Decrease of pH was noticed (from ~6.0 to ~1.5) due to the formation of some
acidic species. The protection monolayer on the cluster core, replaced by chloride ions, led
to destabilization of clusters. As a consequence of these events, silver from cluster/NPs
reacted with chloride ions to form more stable AgCl crystals.
Trichloroethene (TCE) is one of the commonly found organic compounds in groundwa-
ter and is carcinogenic. Advanced materials such as Pd-on-Au bimetallic NPs were used as
catalysts for TCE hydrodechlorination (HDC) in the presence of hydrogen.
46
The reaction
was done at room temperature in water and the product was ethane. Bimetallic NPs were
shown to be more eficient catalysts than monometallic Pd NPs and Pd black (bulk). NPs
were immobilized on silica, alumina, and magnesia to produce active oxide-supported
catalysts. For Pd NPs, the higher activity was attributed to the availability of a greater
amount of coordinatively unsaturated Pd atoms located on surface defects. In the case of
bimetallic systems, Au NPs may be promoting the Pd activity through electronic or geo-
metric effects, or through direct participation in the HDC reaction mechanism. The TCE
HDC reaction was modeled as a Langmuir-Hinshelwood mechanism involving competi-
tive chemisorption of dihydrogen and TCE for all three NP compositions (Pd/Au NPs with
30% and 60% Pd surface coverages, and pure Pd NPs). The HDC reaction was irst-order
for Pd/Au NPs and of non-irst-order for Pd NPs. These differences were attributed to the
differences in sorption afinities for reactant molecules. Recently, Pretzer et al
.
47
found
the variation of the HDC reaction rate with size of the Au particles and coverage of Pd.
Maximum activity was observed for 7 nm Au particles with 60%-70% Pd coverage.
26.2.3.3 Other Organic Compounds
There are many organic contaminants, such as toluene- and sulfur-containing odorous mol-
ecules (e.g., thiophenol, thioether, thioanisole, and sulides), present in water that cause its