Environmental Engineering Reference
In-Depth Information
were supported on neutral alumina. These materials were packed in columns and were
used for the complete removal of pesticides from water passing through the columns.
On this basis, Pradeep and colleagues
38-40
have demonstrated a nanotechnology-based
water puriier for the irst time in the world. Detection limits of CP and malathion were
enhanced to parts-per-billion levels by the addition of Na
2
SO
4
to the Au@citrate NPs.
41
Addition of Na
2
SO
4
to the as-prepared Au particles brought change in color from wine red
to purple along with small decrease in SPR peak intensity and a slight red shift. To the Au
NPs + Na
2
SO
4
mixture, different concentrations of CP solutions were added. The purple
color of the mixture has changed at 25 ppb CP to a blue color in which the SPR peak inten-
sity decreased and a new peak emerged around 680 nm. The intensity of the 680 nm peak
further red-shifted with the increase of CP concentration. Red shift and blue color were
attributed to aggregation of NPs due to adsorption of CP on the NP surface. Similar obser-
vations were made with malathion. Other salts such as NaCl, K
2
SO
4
, and (NH
4
)
2
SO
4
were
also tested to identify the detection limit. It was found that in the presence of Na
2
SO
4
, the
detection limit was quite low. With this method, CP and malathion were detected down to
10.8 and 40.3 ppb, respectively.
It is very important to understand the nature of interactions between the surface of NPs
and the pesticide molecules. The most important interaction is adsorption. The surface
chemistry of NPs and pesticides is yet to be understood. There is a great concern about
the impact of pesticide molecules and their degradation products/metabolites on human
health. Some molecules, such as CP, show degradation of their chemical structure even
in the absence of irradiation of light such as UV (which is generally used for degrada-
tion of organic molecules). CP undergoes hydrolysis in the presence of silver NPs to give
3,5,6-trichloro-2-pyridinol (TCP), which is less toxic compared with CP and diethyl thio-
phosphate.
42
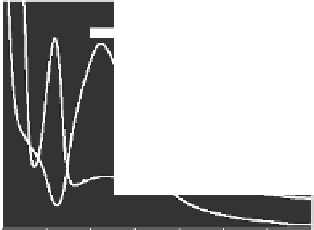
Aggregation of NPs was conirmed by TEM analysis and was relected in the
red shift of the SPR peak (Figure 26.5a). A new peak at 320 nm in the absorption spectrum
of the reaction solution due to TCP was noticed. Formation of TCP was conirmed by elec-
trospray ionization mass spectrometry (ESI MS). The molecular ion peak of CP at m/z
(mass-to-charge ratio) 350 was completely absent after the reaction (Figure 26.5b). A new
(a)
(b)
320
0.6
422
220
198
0.4
50 nm
b
350
198
660
660
0.2
b
a
a
0.0
200
400
600
800
100
200
300
Wavelength (nm)
m/z
FIGURE 26.5
(a) UV/Vis absorption spectra of Ag@citrate NPs treated with 1 and 50 ppm of CP (traces
a
and
b
, respectively)
for 24 h. Inset: TEM image of Ag NPs treated with CP. (b) ESI MS of parent CP and reaction product of CP + Ag
NPs (traces
a
and
b
, respectively) in positive mode. Absence of the molecular ion peak of CP at m/z 350 in trace
b
is marked. (Adapted from Bootharaju, M.S., and Pradeep, T.,
Langmuir
, 28, 2671, 2012. Copyright with permission
from American Chemical Society.)