Environmental Engineering Reference
In-Depth Information
(a)
(b)
pH 3.0
pH 4.5
1.5
Pb
2+
1.0
200 nm
1.0
Pb
2+
0.5
0.5
0.
400
0.0
800
Wavelength (nm)
600
500
600
700
800
Wavelength (nm)
(c)
pH 4.5
Pb
2+
1.0
100 nm
0.5
0.0
400
500
600
700
Wavelength (nm)
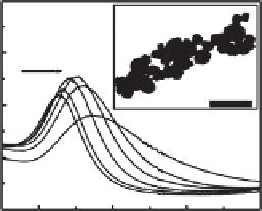
FIGURE 26.3
Absorption spectra of Au/Ag NPs in water after addition of increasing amounts (0-150 μM) of Pb
2+
ions. (a and
b) Effect of Pb
2+
ions on Au NPs at pH 3.0 and 4.5, respectively. (c) Effect of Pb
2+
ions on Ag NPs at pH 4.5. Insets
show TEM images of Au (b) and Ag (c) NPs in the presence of 100 μM Pb
2+
ions. (Adapted from Yoosaf, K. et al.,
J. Phys. Chem. C
., 111, 12839, 2007. Copyright with permission from American Chemical Society.)
by luorescence quenching method were in good agreement with concentrations obtained
in inductively coupled plasma-mass spectrometry data. Copper clusters encapsulated
in BSA proteins were also used to selectively detect Pb
2+
ions in ppm concentrations.
22
Luminescence of the clusters was quenched with an increase in concentration of Pb
2+
. The
reason for quenching of luminescence was obtained from dynamic light scattering (DLS)
measurements. In DLS, aggregation of many clusters due to the interaction of functional
groups of protein with Pb
2+
ions was noticed.
26.2.1.3 Copper
Although copper is one of the essential elements, beyond a certain limit (>1.3 ppm in drinking
water), it is toxic. Several colorimetric and luorescence sensors have been developed in the
recent past. Red luminescence of cyclodextrin (CD)-encapsulated Au
15
clusters protected with
GSH ligands was selectively quenched by Cu
2+
.
23
The reason for quenching of luminescence
was understood from x-ray photoelectron spectroscopy (XPS) analysis in which copper was
found to be in the zerovalent state (Cu
0
). The reduction of Cu
2+
to Cu
0
was possible due to the
enhanced reactivity at a size level of QCs. The cluster core gives electrons for the reduction of
Cu
2+
, and the reduced copper stays on the surface of reacted clusters. As a result of the change
in chemical environment, luorescence was quenched. For practical applications, the cluster
was incorporated into the chitosan ilm and was used for copper sensing similar to a pH
paper strip. Red-luorescent gold NPs, protected with MUA, were used to selectively sense