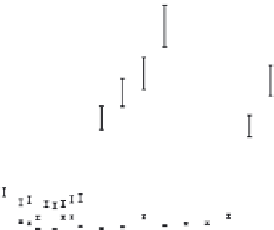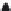Environmental Engineering Reference
In-Depth Information
conirmed by x-ray diffraction (XRD) and energy-dispersive x-ray spectrometry analyses.
Ojea-Jimenez et al
.
13
demonstrated the use of Au@citrate NPs of 8.9 ± 1.6 nm size for
sequestration of Hg
2+
ions from Milli-Q and real waters. They have proposed that the sur-
face of gold NPs catalyze the reduction of Hg
2+
by citrate groups that are present on their
surfaces. The formation of an Au
3
Hg alloy was identiied. Gold may be recovered from the
alloy. Mercaptosuccinic acid (MSA)-protected Ag NPs of different sizes were studied for
Hg
2+
sorption.
Sumesh et al
.
14
have synthesized Ag@MSA NPs of sizes 9 ± 2 nm and 20 ± 5 nm for
which Ag/MSA mole ratios was maintained as 1:6 and 1:3, respectively (Figure 26.2a).
These NPs were supported on alumina, and they were used for Hg
2+
removal in column
setups. A solution of 2 ppm Hg
2+
was passed in separate columns containing 1:3 and 1:6
Ag@MSA materials at identical low rates. Mercury was detected in the eluent after pass-
ing 2.0 and 5.5 L Hg
2+
solutions in the case of 1:3 and 1:6 Ag@MSA, respectively (Figure
26.2b). Concentration of Hg
2+
went below 85 ppb (in the case of 1:6 Ag@MSA) with an input
concentration of 2 ppm. A high removal ability of 800 mg Hg/g Ag@MSA was achieved in
the case of 1:6 Ag@MSA. From this, it is clear that small NPs (1:6 Ag@MSA) adsorb larger
quantities of mercury owing to the presence of a larger number of functional groups per
unit mass compared with NPs of a bigger size.
Gold and silver QCs were shown to have an ability to detect Hg
2+
ions down to 2 ppb
level. This concentration is the maximum contamination limit in drinking water set by
the US EPA. Xie et al
.
15
used red-emitting Au
25
clusters encapsulated in a protein matrix
(bovine serum albumin [BSA]) for highly selective detection of Hg
2+
. Here, detection has
been on the basis of quenching of the PL of encapsulated clusters due to a high degree of
Hg
2+
-Au
+
(d
10
-d
10
) interactions. The detection limit was 0.5 nM (0.1 ppb). Luminescence
had partially been regained after reducing Hg
2+
to Hg
0
by an NaBH
4
solution. Speciic d
10
-
d
10
interactions were noticed in the Ag
+
-Cu
+
cases also. For this, they had prepared core-
shell Au@Ag clusters by reducing Ag
+
ions on as-prepared Au
25
BSA clusters. Introduction
of Cu
2+
ion quenches the luminescence of the Au@AgBSA clusters due to Ag
+
-Cu
+
metallo-
philic interactions. Here, formation of Cu
+
was by the reduction of Cu
2+
by BSA molecules.
(a)
(b)
-1:6 Ag@MSA
-1:3 Ag@MSA
1500
1200
900
600
300
0
01
2
34567
50 nm
Volume passed (L)
FIGURE 26.2
(a) TEM image of parent 1:6 Ag@MSA NPs. (b) Plot of the concentration of Hg
2+
detected in the eluent as a func-
tion of the volume of Hg
2+
solution passed through individual columns packed with 3.0 g each of 1:3 and 1:6
Ag@MSA NPs supported on alumina. Loading of 1:6 and 1:3 Ag@MSA NPs on alumina was 0.5 and 0.3 wt%,
respectively. (Adapted from Sumesh, E. et al.,
J. Hazard. Mater
., 189, 450, 2011. Copyright with permission from
Elsevier.)