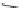Environmental Engineering Reference
In-Depth Information
OH
OH
OH
O
OH
O
OH
OH
O
O
O
OH
OH
n
OH
FIGURE 20.7
Chemical structure of cellulose.
20.4.1 Preparation of Cellulose and Chitosan Nanofibers
20.4.1.1 Cellulose Nanoibers
Traditionally, ibers from cellulose are formed through wet spinning of bleached wood
pulp. The pulp is then dissolved in
N
-methylmorpholine
N
-oxide and passed through spin-
nerets to form microscale ibers. In many respects, the formation of nano- and microscale
ibers from cellulose via electrospinning has mirrored the history of conventional cellu-
lose iber spinning.
66
This is because solubilization of cellulose without derivatization has
posed challenges. Electrospinning of cellulose (Figure 20.7) has been achieved through
the use of readily soluble cellulose acetate and reforming it through treatment with a
strong alkaline solution. The optimized conditions for preparation of nanoibers include
16% (w/v) polymer solution, prepared in acetone/
N
,
N
-dimethylacetamide solvent system,
a 20-gauge (0.45 mm bore diameter) stainless-steel hypodermic needle, a grounded alumi-
num collector at 14 cm tip-to-collector distance, and an applied high-voltage direct current
current of 15 kV. A programmable syringe pump (NE-1000 single syringe pump, New
Era Pump Systems Inc.) aids to pump the solution through and maintain a constant low
rate; otherwise, a glass pipette tilted at an angle can be used. Residual solvent needs to be
removed before the ibers are peeled off from the foil. This is done by evaporation by heat-
ing the nanoiber mat in an oven at 200°C for 1 h.
Cellulose nanoibers are regenerated through deacetylation of the nonwoven iber mat
by soaking it in 0.3 M NaOH solution for 8 h followed by washing with double-distilled
water to obtain neutral pH.
20.4.1.2 Chitin and Chitosan Nanoibers
Electrospinning of pure chitosan solutions poses many challenges
6 7, 6 8
because of their
intractable molecular structure. Chitosan is a positively charged polyelectrolyte at pH 2-6
due to free amino groups, which contribute to its higher solubility in comparison with
chitin. However, this property makes chitosan solutions highly viscous and complicates
their electrospinning.
69
Furthermore, the formation of strong hydrogen bonds in a three-
dimensional network prevents the movement of polymeric chains exposed to the electrical
ield. Successful electrospun chitosan nanoibers from highly concentrated solutions have
been reported.
70,71
Schiffman and Schauer
72
have observed that chitosan has a high solubil-
ity in polar aprotic solvents such as acetic acid and triluoroacetic acid. Figure 20.8a and b
show the chemical structures for chitin and chitosan, respectively.
Owing to the above challenges, blending of chitosan polymer with polyacrylamide
(PAM) in the ratio of 7.7:2.3 (w/w) and dissolving in 60% acetic acid at 90°C yields an elec-
trospinnable polymer solution. The optimized electrospinning parameters are as follows:
low rate, 0.60 mL/h; tip-to-collector distance, 19 cm; and applied voltage, 20 kV.