Environmental Engineering Reference
In-Depth Information
Phosphonic acid
O
P
O
O
C
O
OH
Carboxylic acid
O
O
O
Si
O
O
O
Trimethoxy silane
O
O
O
Si
Dopamine
O
O
Amine
C
O
O
S
S
C
Cysteine
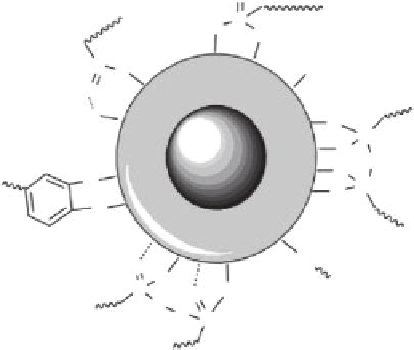
FIGURE 14.8
Common chemical moieties for the anchoring of polymers and functional groups at the surface of iron oxide
MNPs. (From Dias A, Hussain A, Marcos AS et al.,
Biotechnology Advances
, 29, 142, 2011.)
[154]. For example, the removal of heavy metal ions (such as Pb
2+
and Hg
2+
) from industrial
wastewater by monodispersed Fe
3
O
4
-silica core-shell microspheres refers to surface site
binding, i.e., the interactions between SiO
2
and heavy metal ions [141]. Such an adsorption
process is reversible and the binding ions can be desorbed from SiO
2
in weak acidic water
solution under ultrasound radiation. The removal of heavy metal ions (Cd
2+
, Zn
2+
, Pb
2+
, and
Cu
2+
) using MNPs modiied with 3-aminopropyltriethoxysilane (APS) and copolymers
of acrylic acid (AA) and crotonic acid (CA) rely on the electrostatic interactions between
−COO
−
and M
2+
(Figure 14.9) [157]. Adsorption of heavy metals (Cu
2+
, Cd
2+
, Pb
2+
, and Hg
2+
)
by Fe
3
O
4
MNPs coated with humic acid also fall into this kind of mechanism, in which
the electrostatic interactions between −COO
−
of humic acids and M
2+
contribute partly
to the adsorption [158]. To selectively and effectively remove heavy metals from aqueous
solutions, mercapto and amine groups have often been proposed as important functional
groups for synthesizing MNMs of high quality owing to their stronger ligand combinations
on certain heavy metal ions. Thiol-containing polymer-encapsulated MNPs can effectively
2+
M
-
OOC
HOOC
-
OOC
HOOC
HN
2+
M
-
OOC
-
OOC
2+
M
-
OOC
O
HN
HN
Metal ions (M
2+
)
COOH
O
2+
M
O
H
pH > pH
pzc
O -
O -
M
2+
2+
M
M
2+
FIGURE 14.9
Schematic of the possible mechanism for adsorption of metal ions by Fe
3
O
4
@APS@AA-co-CA. (From Ge F, Li
MM, Ye H et al.,
Journal of Hazardous Materials
, 211, 366, 2012.)