Environmental Engineering Reference
In-Depth Information
characteristic features. Polymers are convenient to use and do not affect the pH of the
medium. The addition of polymers in parts per million (ppm) amounts can drastically
change the degree of aggregation of cells, and thereby are cost effective. Large tonnage
use of inorganic compounds (alum and FeCl
3
) produces large amounts of sludge—a prob-
lem absent when polymeric locculants are used.
5
For optimal operation, the choice of
an appropriate polymer is important. In addition, increasing the molecular weight of the
polymer improves locculation, probably by promoting bridge formation. Two main mech-
anisms of locculation are proposed: (a) formation of macromolecular bridges between the
particles, and (b) formation of the surface potential and charge reduction due to adsorp-
tion of highly charged polyelectrolytes on oppositely charged particles.
4
These organic polymeric locculants fall into two categories, namely natural and syn-
thetic polymers. The polysaccharides, mainly starch and its derivatives, different types
of gums, alginic acid, cellulose and its derivatives, dextran, glycogen, chitosan, etc., are
among the natural polymers used in locculation. Synthetic locculants are broadly divided
into anionic, cationic, and nonionic categories. Polyacrylamide (PAM) and poly(ethylene
oxide) are nonionic. Cationic groups of polyelectrolytes are derived by introducing qua-
ternary ammonium groups onto the polymer backbone. In addition to that, sulfonium
and phosphonium groups are used to a limited extent. The most commonly used cationic
polyelectrolyte is poly(diallyl dimethyl ammonium chloride). In the group of anionic poly-
electrolytes, mainly two types of polymers are used; one type is polymers containing car-
boxyl functional groups and the other containing sulfonic acid groups. A representative of
the former is poly(acrylic acid) (PAA) and its derivatives, of the latter polystyrene sulfonic
acid.
9
Among polymeric locculants, the synthetic polymers can be made by controlling
the molecular weight, molecular weight distribution, and chemical structure. Thus, due to
tailorability, synthetic polymers can be very eficient locculants. Structures of some of the
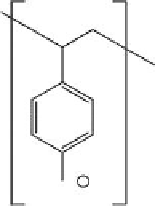
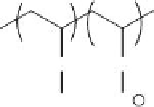
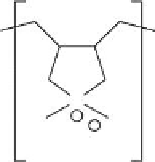
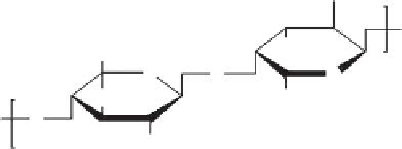
polymers used for removal of pathogens are shown below (Figure 12.1).
NH
2
(a)
(b)
OH
OH
n
OH
CH
2
OH
CH
2
OH
n
O
O
O
O
O
O
CH
2
OH
OH
CH
2
OH
OH
O
O
NH
2
OH
(f )
(c)
(d)
(e)
m
n
m
n
CO
CO
CO
CO
N
+
NH
2
O
+
-
-
-
NH
2
SO
3
NMe
3
n
Cl
n
FIGURE 12.1
Representative structures of nonionic, anionic, and cationic polymers used in locculation: (a) cellulose; (b) chi-
tosan; (c) poly(diallyl dimethyl ammonium chloride), poly(DADMAC); (d) cationic polyacrylamide; (e) anionic
polyacrylamide; (f) polystyrene sulfonate.