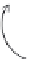Environmental Engineering Reference
In-Depth Information
Red
2
-2.0
Reduction site
-1.0
E
C
e
hv
Ox
2
0
1.0
Red
1
2.0
Photocatalyst
h
E
V
3.0
Ox
1
Oxidation site
4.0
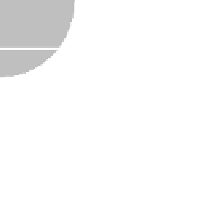
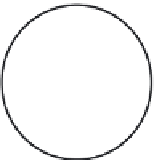
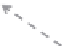
FIGURE 11.12
(See color insert.)
Schematic diagram illustrating the reaction process of cocatalyst-modiied semiconductor
photocatalyst.
MoS
2
as a cocatalyst coupled with CdS exhibited a better photoreactivity than a Pt-loaded
sample for H
2
evolution.
50
The oxidation reaction is usually considered as the rate-determining process in the pho-
tocatalytic reaction. Coupling a suitable cocatalyst to reduce the overpotential of the oxida-
tion reaction will greatly accelerate the overall photocatalytic process. Metal oxides, such
as IrO
2
, RuO
2
, and cobalt phosphate, have been shown to promote oxidation reactions.
Furthermore, codeposition of Pt as a reduction cocatalyst and IrO
2
as an oxidation cocata-
lyst on TiO
2
demonstrated additional enhancement of the reduction and oxidation pro-
cesses.
51
Thus, the design and development of new types of cocatalysts play a signiicant
role in enhancing photocatalytic eficiency.
11.3.6 Plasmon-Exciton Coupling
Surface plasmon resonance (SPR) of metallic nanostructure can be described as the res-
onant photon-induced collective oscillation of valance photons that subsequently gener-
ate a spatially nonhomogeneous oscillating electronic ield in the neighboring region of
the nanostructure.
52
By manipulating the composition, size, and morphology of metal
nanoparticles, the SPR frequency of metal nanoparticles can be tuned throughout the
entire solar spectrum. The investigations have demonstrated that SPR plays an important
role in enhancing the rate of photocatalytic reactions of the semiconductor in plasmonic
metal-semiconductor composite materials. The highest rate enhancement was observed
at the wavelength corresponding to the metal SPR, which reveals that hybrid structures of
plasmonic metal nanoparticles and semiconductors can induce plasmon-exciton coupling
interactions, which then participate in photochemical processes. There are two nonmu-
tually exclusive mechanisms of excited plasmonic nanostructures in the ield of photo-
chemical reactions: (i) SPR excited direct charge injection from metal to semiconductor, and
(ii) plasmonic electromagnetic ield induced enhancement of the photochemical reaction rates.
One of the important behaviors of plasmonic pumping in metal nanoparticles is non-
radiative decay by the generation of electron-hole pairs and then transfer of the energetically
excited electrons to the CB of the nearby semiconductor (Figure 11.13a). Oxidation and reduc-
tion reactions may occur on the metal and semiconductor, respectively. This is similar to the
dye-sensitization process. The excellent mobility of charge carriers and high absorption cross
section of a metal plasmonic nanostructure make it a promising sensitizer over the entire
solar spectrum. For example, Au/TiO
2
shows a notable visible light photoreactivity for water